Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood
- PMID: 26836199
- PMCID: PMC7073407
- DOI: 10.1002/14651858.CD007786.pub3
Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood
Abstract
Background: Nausea and vomiting remain a problem for children undergoing treatment for malignancies despite new antiemetic therapies. Optimising antiemetic regimens could improve quality of life by reducing nausea, vomiting, and associated clinical problems. This is an update of the original systematic review.
Objectives: To assess the effectiveness and adverse events of pharmacological interventions in controlling anticipatory, acute, and delayed nausea and vomiting in children and young people (aged less than 18 years) about to receive or receiving chemotherapy.
Search methods: Searches included the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, PsycINFO, conference proceedings of the American Society of Clinical Oncology, International Society of Paediatric Oncology, Multinational Association of Supportive Care in Cancer, and ISI Science and Technology Proceedings Index from incept to December 16, 2014, and trial registries from their earliest records to December 2014. We examined references of systematic reviews and contacted trialists for information on further studies. We also screened the reference lists of included studies.
Selection criteria: Two review authors independently screened abstracts in order to identify randomised controlled trials (RCTs) that compared a pharmacological antiemetic, cannabinoid, or benzodiazepine with placebo or any alternative active intervention in children and young people (less than 18 years) with a diagnosis of cancer who were to receive chemotherapy.
Data collection and analysis: Two review authors independently extracted outcome and quality data from each RCT. When appropriate, we undertook meta-analysis.
Main results: We included 34 studies that examined a range of different antiemetics, used different doses and comparators, and reported a variety of outcomes. The quality and quantity of included studies limited the exploration of heterogeneity to narrative approaches only.The majority of quantitative data related to the complete control of acute vomiting (27 studies). Adverse events were reported in 29 studies and nausea outcomes in 16 studies.Two studies assessed the addition of dexamethasone to 5-HT3 antagonists for complete control of vomiting (pooled risk ratio (RR) 2.03; 95% confidence interval (CI) 1.35 to 3.04). Three studies compared granisetron 20 mcg/kg with 40 mcg/kg for complete control of vomiting (pooled RR 0.93; 95% CI 0.80 to 1.07). Three studies compared granisetron with ondansetron for complete control of acute nausea (pooled RR 1.05; 95% CI 0.94 to 1.17; 2 studies), acute vomiting (pooled RR 2.26; 95% CI 2.04 to 2.51; 3 studies), delayed nausea (pooled RR 1.13; 95% CI 0.93 to 1.38; 2 studies), and delayed vomiting (pooled RR 1.13; 95% CI 0.98 to 1.29; 2 studies). No other pooled analyses were possible.Narrative synthesis suggests that 5-HT3 antagonists are more effective than older antiemetic agents, even when these agents are combined with a steroid. Cannabinoids are probably effective but produce frequent side effects.
Authors' conclusions: Our overall knowledge of the most effective antiemetics to prevent chemotherapy-induced nausea and vomiting in childhood is incomplete. Future research should be undertaken in consultation with children, young people, and families that have experienced chemotherapy and should make use of validated, age-appropriate measures. This review suggests that 5-HT3 antagonists are effective in patients who are to receive emetogenic chemotherapy, with granisetron or palonosetron possibly better than ondansetron. Adding dexamethasone improves control of vomiting, although the risk-benefit profile of adjunctive steroid remains uncertain.
Conflict of interest statement
RSP: no financial conflicts of interest
AJF: no financial conflicts of interest
FG: no financial conflicts of interest
EH: no financial conflicts of interest
SG: no financial conflicts of interest
JVC: no financial conflicts of interest
BP: no financial conflicts of interest
Figures

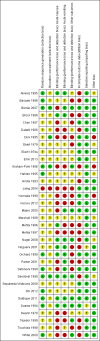






Update of
-
Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood.Cochrane Database Syst Rev. 2010 Sep 8;(9):CD007786. doi: 10.1002/14651858.CD007786.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Feb 02;2:CD007786. doi: 10.1002/14651858.CD007786.pub3. PMID: 20824866 Updated.
Similar articles
-
Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood.Cochrane Database Syst Rev. 2010 Sep 8;(9):CD007786. doi: 10.1002/14651858.CD007786.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Feb 02;2:CD007786. doi: 10.1002/14651858.CD007786.pub3. PMID: 20824866 Updated.
-
Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD012775. doi: 10.1002/14651858.CD012775.pub2. Cochrane Database Syst Rev. 2021. PMID: 34784425 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults.Cochrane Database Syst Rev. 2018 Sep 21;9(9):CD012555. doi: 10.1002/14651858.CD012555.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246876 Free PMC article.
-
Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy.Cochrane Database Syst Rev. 2015 Nov 12;2015(11):CD009464. doi: 10.1002/14651858.CD009464.pub2. Cochrane Database Syst Rev. 2015. PMID: 26561338 Free PMC article.
Cited by
-
Effect of aromatherapy with rose essential oil on the nausea and vomiting in chemotherapy patients: a randomized controlled trial.Ann Med Surg (Lond). 2023 Nov 16;86(1):225-231. doi: 10.1097/MS9.0000000000001395. eCollection 2024 Jan. Ann Med Surg (Lond). 2023. PMID: 38222701 Free PMC article.
-
External Application of Traditional Chinese Medicine in the Prevention and Treatment of Nausea and Vomiting Caused by Chemotherapy of Non-Small-Cell Lung Cancer: A RCT Study.Evid Based Complement Alternat Med. 2022 Aug 4;2022:6992673. doi: 10.1155/2022/6992673. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 6;2023:9810509. doi: 10.1155/2023/9810509. PMID: 35966745 Free PMC article. Retracted.
-
Development of a web-based assessment tool that evaluates the meal situation when a child has a percutaneous endoscopic gastrostomy.BMC Pediatr. 2019 Mar 11;19(1):76. doi: 10.1186/s12887-019-1447-1. BMC Pediatr. 2019. PMID: 30857527 Free PMC article.
-
The Immunomodulatory Effects of Dexamethasone on Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer.Oncol Ther. 2023 Sep;11(3):361-374. doi: 10.1007/s40487-023-00235-6. Epub 2023 Jun 24. Oncol Ther. 2023. PMID: 37354381 Free PMC article.
-
The Use of Complementary and Integrative Medicine in Combination With Pharmacological Antiemetics to Address Chemotherapy-Induced Nausea and Vomiting in Pediatric Oncology: A Scoping Review.J Pediatr Hematol Oncol Nurs. 2024 Sep-Oct;41(5):358-369. doi: 10.1177/27527530241267294. Epub 2024 Aug 28. J Pediatr Hematol Oncol Nurs. 2024. PMID: 39197861
References
References to studies included in this review
Alvarez 1995 {published data only}
-
- Alvarez O, Freeman A, Bedros A, Call SK, Volsch J, Kalbermatter O, et al. Randomized double‐blind crossover ondansetron‐dexamethasone versus ondansetron‐placebo study for the treatment of chemotherapy‐induced nausea and vomiting in pediatric patients with malignancies. Journal of Pediatric Hematology/Oncology 1995;17(2):145‐50. - PubMed
Basade 1996 {published data only}
-
- Basade M, Kulkarni SS, Dhar AK, Sastry PS, Saikia B, Advani SH. Comparison of dexamethasone and metoclopramide as antiemetics in children receiving cancer chemotherapy. Indian Pediatrics 1996;33(4):321‐3. - PubMed
Berrak 2007 {published data only (unpublished sought but not used)}
-
- Berrak SG, Ozdemir N, Bakirci N, Turkkan E, Canpolat C, Beker B, et al. A double‐blind, crossover, randomized dose‐comparison trial of granisetron for the prevention of acute and delayed nausea and emesis in children receiving moderately emetogenic carboplatin‐based chemotherapy. Supportive Care in Cancer 2007;15(10):1163‐8. - PubMed
Brock 1996 {published data only}
-
- Brock P, Brichard B, Rechnitzer C, Langeveld NE, Lanning M, Soderhall S, et al. An increased loading dose of ondansetron: a north European, double‐blind randomised study in children, comparing 5 mg/m2 with 10 mg/m2. European Journal of Cancer 1996;32A(10):1744‐8. - PubMed
Chan 1987 {published data only}
-
- Chan HS, Correia JA, MacLeod SM. Nabilone versus prochlorperazine for control of cancer chemotherapy‐induced emesis in children: a double‐blind, crossover trial. Pediatrics 1987;79(6):946‐52. - PubMed
Dalzell 1986 {published data only}
Dick 1995 {published data only}
Ekert 1979 {published data only}
-
- Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy‐induced nausea and vomiting by delta‐9‐tetrahydrocannabinol. Medical Journal of Australia 1979;2:657‐9. - PubMed
Ekert 1979a {published data only}
-
- Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy‐induced nausea and vomiting by delta‐9‐tetrahydrocannabinol. Medical Journal of Australia 1979;2(12):657‐9. - PubMed
Emir 2013 {published data only}
Graham‐Pole 1986 {published data only}
-
- Graham‐Pole J, Weare J, Engel S, Gardner R, Mehta P, Gross S. Antiemetics in children receiving cancer chemotherapy: a double‐blind prospective randomized study comparing metoclopramide with chlorpromazine. Journal of Clinical Oncology 1986;4(7):1110‐3. - PubMed
Hahlen 1995 {published data only}
-
- Hahlen K, Quintana E, Pinkerton CR, Cedar E. A randomized comparison of intravenously administered granisetron versus chlorpromazine plus dexamethasone in the prevention of ifosfamide‐induced emesis in children. Journal of Pediatrics 1995;126(2):309‐13. - PubMed
Hirota 1993 {published data only}
-
- Hirota T, Honjo T, Kuroda R, Saeki K, Katano N, Sakakibara Y, et al. Antiemetic efficacy of granisetron in pediatric cancer treatment ‐ (2). Comparison of granisetron and granisetron plus methylprednisolone as antiemetic prophylaxis. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1993;20(15):2369‐73. - PubMed
Jaing 2004 {published and unpublished data}
-
- Jaing T‐H, Tsay P‐K, Hung I‐J, Yang C‐P, Hu W‐Y. Single‐dose oral granisetron versus multidose intravenous ondansetron for moderately emetogenic cyclophosphamide‐based chemotherapy in pediatric outpatients with acute lymphoblastic leukemia. Pediatric Hematology & Oncology 2004;21(3):227‐35. - PubMed
Komada 1999 {published data only}
-
- Komada Y, Matsuyama T, Takao A, Hongo T, Nishimura Y, Horibe K, et al. A randomised dose‐comparison trial of granisetron in preventing emesis in children with leukaemia receiving emetogenic chemotherapy. European Journal of Cancer 1999;35(7):1095‐101. - PubMed
Kurucu 2012 {published data only}
-
- Kurucu N, Durmaz M. Effect of hydroxyzine in controlling acute chemotherapy‐induced vomiting in children: a randomised trial. UHOD ‐ Uluslararasi Hematoloji‐Onkoloji Dergisi 2012;22(2):99‐106.
Mabro 2000 {published data only}
-
- Mabro M, Cohn R, Zanesco L, Madon E, Hahlen K, Margueritte G, et al. Oral granisetron solution as prophylaxis for chemotherapy‐induced emesis in children: double‐blind study of 2 doses. Bulletin du Cancer 2000;87(3):259‐64. - PubMed
Marshall 1989 {published data only}
-
- Marshall G, Kerr S, Vowels M, O'Gorman‐Hughes D, White L. Antiemetic therapy for chemotherapy‐induced vomiting: metoclopramide, benztropine, dexamethasone, and lorazepam regimen compared with chlorpromazine alone. Journal of Pediatrics 1989;115(1):156‐60. - PubMed
Mehta 1986 {published data only}
-
- Mehta P, Gross S, Graham‐Pole J, Gardner R. Methylprednisolone for chemotherapy‐induced emesis: a double‐blind randomized trial in children. Journal of Pediatrics 1986;108(5 Pt 1):774‐6. - PubMed
Mehta 1997 {published data only}
-
- Mehta NH, Reed CM, Kuhlman C, Weinstein HJ, Parsons SK. Controlling conditioning‐related emesis in children undergoing bone marrow transplantation. Oncology Nursing Forum 1997;24(9):1539‐44. - PubMed
Nagel 2008 {published data only}
-
- Nagel K, Willan AR, Lappan J, Korz L, Buckley N, Barr RD. Pediatric Oncology Sedation Trial (POST): a double‐blind randomized study. Pediatric Blood and Cancer 2008;51:634‐8. - PubMed
Noguera 2001 {published data only}
-
- Noguera D, Gómez R, Marcano A. 5‐hydroxytriptamine type 3 receptor antagonists in the prevention of vomiting by cytostatics drugs in children [Antagonistas de receptores 5‐hidroxitriptamina tipo 3 en la prevención de emesis por citostáticos en niños]. Salus Militiae 2001;26(1):71‐6.
Orchard 1999 {published data only (unpublished sought but not used)}
-
- Orchard PJ, Rogosheske J, Burns L, Rydholm N, Larson H, DeFor TE, et al. A prospective randomized trial of the anti‐emetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biology of Blood & Marrow Transplantation 1999;5(6):386‐93. - PubMed
Parker 2001 {published data only}
-
- Parker RI, Prakash D, Mahan RA, Giugliano DM, Atlas MP. Randomized, double‐blind, crossover, placebo‐controlled trial of intravenous ondansetron for the prevention of intrathecal chemotherapy‐induced vomiting in children. Journal of Pediatric Hematology/Oncology 2001;23(9):578‐81. - PubMed
Safonova 1999 {published data only}
-
- Safonova SA, Gershanovich ML, Punanov IuA, Kolygin BA. Evaluation of the anti‐emetic effectiveness of two drug formulations of ondansetron in combined chemotherapy for children with malignant tumors. Voprosy Onkologii 1999;45(4):424‐8. - PubMed
Sandoval 1999 {published data only}
-
- Sandoval C, Corbi D, Strobino B, Ozkaynak MF, Tugal O, Jayabose S. Randomized double‐blind comparison of single high‐dose ondansetron and multiple standard‐dose ondansetron in chemotherapy‐naive pediatric oncology patients. Cancer Investigation 1999;17(5):309‐13. - PubMed
Sepulveda‐Vildosola 2008 {published data only}
-
- Sepulveda‐Vildosola AC, Betanzos‐Cabrera Y, Lastiri GG, Rivera‐Marquez H, Villasis‐Keever MA, Angel VW, et al. Palonosetron hydrochloride is an effective and safe option to prevent chemotherapy‐induced nausea and vomiting in children. Archives of Medical Research 2008;39:601‐6. - PubMed
Shi 2012 {published data only}
-
- Shi X, Liu ZM, Zhu XD. Treatment of vomiting in children patients with solid tumor by hewei zhiou recipe combined ondansetron hydrochloride. Chinese Journal of Integrated Traditional and Western Medicine 2012;32(4):468‐70. - PubMed
Siddique 2011 {published data only}
-
- Siddique R, Hafiz MG, Rokeya B, Jamal CY, Islam A. Ondansetron versus granisetron in the prevention of chemotherapy induced nausea and vomiting in children with acute lymphoblastic leukaemia. Mymensingh Medical Journal 2011;20(4):680‐8. - PubMed
Suarez 1994 {published data only}
-
- Suarez A, Stettler ER, Rey E, Pons G, Simonetta‐Chateauneuf C, Bruijn KM, et al. Safety, tolerability, efficacy and plasma concentrations of tropisetron after administration at five dose levels to children receiving cancer chemotherapy. European Journal of Cancer 1994;30A(10):1436‐41. - PubMed
Swann 1979 {published data only}
Tejedor 1999 {published data only}
-
- Tejedor I, Idoate A, Jimenez M, Sierrasesumaga L, Giraldez J. Cost‐effectiveness analysis of tropisetron vs. chlorpromazine‐dexamethasone in the control of acute emesis induced by highly emetogenic chemotherapy in children. Pharmacy World & Science 1999;21(2):60‐8. - PubMed
Tsuchida 1999 {published data only}
-
- Tsuchida Y, Hayashi Y, Asami K, Yamamoto K, Yokoyama J, Mugishima H, et al. Effects of granisetron in children undergoing high‐dose chemotherapy: a multi‐institutional, cross‐over study. International Journal of Oncology 1999;14(4):673‐9. - PubMed
White 2000 {published data only}
-
- White L, Daly SA, McKenna CJ, Zhestkova N, Leal C, Breatnach F, et al. A comparison of oral ondansetron syrup or intravenous ondansetron loading dose regimens given in combination with dexamethasone for the prevention of nausea and emesis in pediatric and adolescent patients receiving moderately/highly emetogenic chemotherapy. Pediatric Hematology & Oncology 2000;17(6):445‐55. - PubMed
References to studies excluded from this review
Aksoylar 2001 {published and unpublished data}
-
- Aksoylar S, Akman SA, Ozgenc F, Kansoy S. Comparison of tropisetron and granisetron in the control of nausea and vomiting in children receiving combined cancer chemotherapy. Pediatric Hematology & Oncology 2001;18(6):397‐406. - PubMed
Alavi 1985 {published data only}
-
- Alavi JB, Torri S, Glover D, Hurwitz S, Glick JH. High‐dose oral metoclopramide. An effective antiemetic agent. American Journal of Clinical Oncology 1985;8(3):260‐5. - PubMed
Basch 2011 {published data only}
Bauduer 1999 {published data only}
-
- Bauduer F, Coiffier B, Desablens B. Granisetron plus or minus alprazolam for emesis prevention in chemotherapy of lymphomas: a randomized multicenter trial. Granisetron Trialists Group. Leukemia & Lymphoma 1999;34(3‐4):341‐7. - PubMed
Bonaventura 1999 {published data only}
-
- Bonaventura A, Freeman S, Stewart J, Ackland S, Hall K, Gebski V. Control of acute and delayed vomiting with lorazepam, dexamethasone and metochlopramide (LDM) versus dexamethasone and ondansetron (DO) following highly emetogenic chemotherapy (meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1999:2299.
Brice 1989 {published data only}
-
- Brice P, Fiere D, Gastaut JL, Simon‐Lejeune C, Carcassonne Y, Tredaniel J, et al. Comparison of the antiemetic effectiveness of high‐dose corticosteroids with synacthene in nausea induced by chemotherapy: results of a randomized study. Bulletin du Cancer 1989;76(6):637‐42. - PubMed
Corapcioglu 2005 {published data only}
-
- Corapcioglu F, Sarper N. A prospective randomized trial of the antiemetic efficacy and cost‐effectiveness of intravenous and orally disintegrating tablet of ondansetron in children with cancer. Pediatric Hematology and Oncology 2005;22(2):103‐14. - PubMed
Dana 1987 {published data only}
-
- Dana BW, McDermott M, Everts E, Abdulhay G. A randomized trial of high‐dose bolus metoclopramide versus low‐dose continuous infusion metoclopramide in the prevention of cisplatin‐induced emesis. American Journal of Clinical Oncology 1987;10(3):253‐6. - PubMed
Delgado 1983 {published data only}
-
- Delgado G, Stefanuto W, Novo NF. Preconous toxicity syndrome due to cancer chemotherapy: therapeutic effects of metoclopramide and corticosteroids compared with placebo [Síndrome da toxidade precoce induzida pela quimioterapia antineoplásica: efeito terapêutico de metoclopramida e corticosteróide versus placebo e proposta de um modelo patogênico]. Acta Oncologica (Brasil) 1983;3(1/3):19‐29.
Fauser 1998 {published data only}
-
- Fauser A, Pizzocaro G, Schuller G, Khayat D, Wilkinson P. A double‐blind parallel study of intravenous (IV) dolasetron + dexamethasone vs. iv dolasetron alone in preventing fractionated cisplatin emesis (meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1998:abstract 223.
Feng 2000 {published data only}
-
- Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Comparison of the selective serotonin antagonists ramosetron and granisetron in treating acute chemotherapy‐induced emesis, nausea, and anorexia: a single‐blind, randomized, crossover study. Current Therapeutic Research ‐ Clinical and Experimental 2000;61(12):901‐9.
Feng 2002 {published data only}
-
- Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Oral formulations of the selective serotonin antagonists ramosetron (intraoral disintegrator formulation) and granisetron hydrochloride (standard tablet) in treating acute chemotherapy‐induced emesis, nausea, and anorexia: a multicenter, randomized, single‐blind, crossover, comparison study. Current Therapeutic Research ‐ Clinical and Experimental 2002;63(11):725‐35.
Forni 2000 {published data only}
-
- Forni C, Ferrari S, Loro L, Mazzei T, Beghelli C, Biolchini A, et al. Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin‐adriamycin and by high‐dose ifosfamide delivered in multiple‐day continuous infusions. Supportive Care in Cancer 2000;8(2):131‐3. - PubMed
Friedman 2000 {published data only}
-
- Friedman CJ, Burris HA, Yocom K, Blackburn LM, Gruben D. Oral granisetron for the prevention of acute late onset nausea and vomiting in patients treated with moderately emetogenic chemotherapy. Oncologist 2000;5(2):136‐43. - PubMed
Gagen 1984 {published data only}
-
- Gagen M, Gochnour D, Young D, Gaginella T, Neidhart J. A randomized trial of metoclopramide and a combination of dexamethasone and lorazepam for prevention of chemotherapy‐induced vomiting. Journal of Clinical Oncology 1984;2(6):696‐701. - PubMed
Gez 1989 {published data only}
-
- Gez E, Ben‐Yosef R, Catane R, Brufman G, Biran S. Chlorpromazine and dexamethasone versus high‐dose metoclopramide and dexamethasone in patients receiving cancer chemotherapy, particularly cis‐platinum: a prospective randomized crossover study. Oncology 1989;46(3):150‐4. - PubMed
Gorena 1996 {published data only}
-
- Gorena M, Giacaman A, Pastor P. Antiemetic therapy in testis cancer chemotherapy: comparison in cross‐over study of ondansetron and metoclopramide [Terapia antiemética en quimioterapia por cáncer testicular: comparación en estudio cross‐over de ondansetron y metoclopramida]. Revista Chile Urologica 1996;61(1):72‐4.
Hatae 1989 {published data only}
-
- Hatae Y, Takeda T, Nakadate H, Hatayama Y, Kishino T, Ogawa Y. The antiemetic effect and clinical evaluation of metoclopramide alone and combined with betamethasone in children with malignant tumor. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1989;16(11):3639‐42. - PubMed
Herrstedt 2007 {published data only}
-
- Herrstedt J, Sigsgaard TC, Nielsen HA, Handberg J, Langer SW, Ottesen S, et al. Randomized, double‐blind trial comparing the antiemetic effect of tropisetron plus metopimazine with tropisetron plus placebo in patients receiving multiple cycles of multiple‐day cisplatin‐based chemotherapy. Supportive Care in Cancer 2007;15(4):417‐26. - PubMed
Hirota 1993a {published data only}
-
- Hirota T, Honjo T, Kuroda R, Saeki K, Katano N, Sakakibara Y, et al. Antiemetic efficacy of granisetron in the treatment of pediatric cancer ‐ (1). Clinical evaluation of granisetron at a dose of 40 micrograms/kg. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1993;20(14):2201‐5. - PubMed
Jara 1999 {published data only}
-
- Jara C, Hernandez M, Alonso C, Fernandez A, Gamez‐Aldarava JL, Mayordomo JI. Dexamethasone with oral granisetron or metoclopramide in preventing chemotherapy‐induced delayed emesis. A randomized, double‐blind trial (meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1999:abstract 2289.
Joss 1994 {published data only}
-
- Joss RA, Bacchi M, Buser K, Kirchner V, Neuenschwander H, Orth B, et al. Ondansetron plus dexamethasone is superior to ondansetron alone in the prevention of emesis in chemotherapy‐naive and previously treated patients. Swiss Group for Clinical Cancer Research (SAKK). Annals of Oncology 1994;5(3):253‐8. - PubMed
Kearsley 1989 {published data only}
-
- Kearsley JH, Williams AM, Fiumara AM. Antiemetic superiority of lorazepam over oxazepam and methylprednisolone as premedicants for patients receiving cisplatin‐containing chemotherapy. Cancer 1989;64(8):1595‐9. - PubMed
Keyhanian 2009 {published data only}
-
- Keyhanian S, Taziki O, Saravi MM, Fotokian Z. A randomized comparison of granisetron plus dexamethason with granisetron alone for the control of acute chemotherapy‐induced emesis and nausea. International Journal of Hematology‐Oncology and Stem Cell Research 2009;3(2):27‐30.
Koseoglu 1998 {published data only}
-
- Koseoglu V, Kurekci AE, Sarici U, Atay AA, Ozcan O. Comparison of the efficacy and side‐effects of ondansetron and metoclopramide‐diphenhydramine administered to control nausea and vomiting in children treated with antineoplastic chemotherapy: a prospective randomized study. European Journal of Pediatrics 1998;157(10):806‐10. - PubMed
Luisi 2006 {published data only}
Matsuoka 2003 {published data only}
-
- Matsuoka S, Okamoto S, Watanabe R, Mori T, Nagayama H, Hamano Y, et al. Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation: a prospective randomized study. International Journal of Hematology 2003;77(1):86‐90. - PubMed
Nathan 2006 {published data only}
-
- Nathan PC, Tomlinson G, Dupuis LL, Greenberg ML, Ota S, Bartels U, et al. A pilot study of ondansetron plus metopimazine vs. ondansetron monotherapy in children receiving highly emetogenic chemotherapy: a Bayesian randomized serial N‐of‐1 trials design. Supportive Care in Cancer 2006;14(3):268‐76. - PubMed
NCT00429702 2007 {published data only}
-
- NCT00429702. Diphenhydramine, lorazepam, and dexamethasone in treating nausea and vomiting caused by chemotherapy in young patients with newly diagnosed cancer. clinicaltrials.gov/show/NCT00429702 (accessed 15 May 2010).
Needles 1999 {published data only}
-
- Needles B, Miranda E, Garcia Rodriguez FM, Diaz LB, Spector J, Craig J, et al. A multicenter, double‐blind, randomized comparison of oral ondansetron 8 mg b.i.d., 24 mg q.d., and 32 mg q.d. in the prevention of nausea and vomiting associated with highly emetogenic chemotherapy. S3AA3012 Study Group. Supportive Care in Cancer 1999;7(5):347‐53. - PubMed
Onat 1995 {published data only}
-
- Onat H, Inanc SE, Saip P, Topuz E. Tropisetron (TRP) in the prevention of emesis associated with 5‐day cisplatin‐based chemotherapy (CT) in patients (pts) with germ cell tumors (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1995:abstract 1760.
Pillai 2011 {published data only}
-
- Pillai AK, Sharma KK, Gupta YK, Bakshi S. Anti‐emetic effect of ginger powder versus placebo as an add‐on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatric Blood and Cancer 2011;56:234‐8. - PubMed
Relling 1993 {published data only}
-
- Relling MV, Mulhern RK, Fairclough D, Baker D, Pui CH. Chlorpromazine with and without lorazepam as antiemetic therapy in children receiving uniform chemotherapy. Journal of Pediatrics 1993;123(5):811‐6. - PubMed
Roila 1995 {published data only}
-
- Roila F, Angelis V, Cognetti F, Ricci S, Contu A, Nuzzo A, et al. Ondansetron vs granisetron, both combined with dexamethasone in the prevention of cisplatin‐induced emesis (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1995:abstract 1721.
Roila 1996 {published data only}
-
- Roila F, Angelis V, Contu A, Scagliotti G, Tateo S, Massidda B, et al. Ondansetron (OND) vs metoclopramide (MTC) both combined with dexamethasone (DEX) in the prevention of cisplatin (CDDP)‐induced delayed emesis (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1996:abstract 1705.
Roila 1998 {published data only}
-
- Roila F, Ballatori E, Contu A, Tateo S, Ricci S, Ionta MT, et al. Optimal dose of intravenous dexamethasone in the prevention of cisplatin‐induced acute emesis: a double blind randomized study. (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1998:abstract 195.
Roila 2000 {published data only}
-
- Roila F, Ballatori E, Bosnjak S, Fava S, Nuzzo A, Contu A, et al. Dexamethasone (DEX) alone or combined with ondansetron (OND) in the prevention of delayed emesis induced by moderately emetogenic chemotherapy (MEC). Proceedings of the American Society of Clinical Oncology 2000;19:(abstr 2358).
Stiakaki 1999 {published data only}
-
- Stiakaki E, Savvas S, Lydaki E, Bolonaki I, Kouvidi E, Dimitriou H, et al. Ondansetron and tropisetron in the control of nausea and vomiting in children receiving combined cancer chemotherapy. Pediatric Hematology & Oncology 1999;16(2):101‐8. - PubMed
Sumer 1988 {published data only}
-
- Sumer T, Abu‐Melha A, Maqbool G, Al‐Mulhim I. Dexamethasone as an antiemetic in children receiving cis‐platinum. American Journal of Pediatric Hematology/Oncology 1988;10(2):126‐8.
Tian 2011 {published data only}
-
- Tian W, Wang Z, Zhou J, Zhang S, Wang J, Chen Q, et al. Randomized, double‐blind, crossover study of palonosetron compared with granisetron for the prevention of chemotherapy‐induced nausea and vomiting in a Chinese population. Medical Oncology 2011;28:71‐8. - PubMed
Tsukuda 2009 {published data only}
-
- Tsukuda M, Ishitoya J, Mikami Y, Matsuda H, Katori H, Horiuchi C, et al. Antiemetic effects of granisetron and dexamethasone combination therapy during cisplatin‐containing chemotherapy for head and neck cancer: dexamethasone dosage verification trial. International Journal of Clinical Oncology 2009;14:337‐43. - PubMed
Yalcin 1999 {published data only}
-
- Yalcin S, Tekuzman G, Baltali E, Ozisik Y, Barista I. Serotonin receptor antagonists in prophylaxis of acute and delayed emesis induced by moderately emetogenic, single‐day chemotherapy: a randomized study. American Journal of Clinical Oncology: Cancer Clinical Trials 1999;22(1):94‐6. - PubMed
Yonemura 2009 {published data only}
-
- Yonemura M, Katsumata N, Hashimoto H, Satake S, Kaneko M, Kobayashi Y, et al. Randomized controlled study comparing two doses of intravenous granisetron (1 and 3 mg) for acute chemotherapy‐induced nausea and vomiting in cancer patients: a non‐inferiority trial. Japanese Journal of Clinical Oncology 2009;39(7):443‐8. - PubMed
References to studies awaiting assessment
Gómez 1995 {published data only}
-
- Gómez MH, Mas LL, Vallejos SC. Tropisetron with dexamethasone in the control of nausea and vomiting in emetogenic chemotherapy [Tropisetron mas dexametasona en el control de náuseas y vómitos en pacientes con quimioterapia emetizante]. Acta Cancerologica 1995;25(2):55‐60.
Xu 1997 {published data only}
-
- Xu B, Zhou J, Zhou A. Phase III clinical studies with ondansetron (Qilu) in the prophylaxis of nausea and vomiting induced by cisplatin. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1997;19(5):358‐61. - PubMed
Zeng 1995 {published data only}
-
- Zeng W, Zhou J, Zhang P. The role of ondansetron (Qilu) in the prevention of non‐cisplatin‐induced vomiting ‐ a randomized clinical trial. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1995;17(4):294‐7. - PubMed
Additional references
Antonarakis 2004a
-
- Antonarakis ES, Evans JL, Heard GF, Noonan LM, Pizer BL, Hain RDW. Prophylaxis of acute chemotherapy‐induced nausea and vomiting in children with cancer: what is the evidence?. Pediatric Blood and Cancer 2004;43(6):651‐8. - PubMed
Antonarakis 2004b
Caldwell 2005
Cipriani 2009
-
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. The Lancet 2009;373(9665):746‐58. - PubMed
Cotanch 1985
-
- Cotanch P, Hockenberry M, Herman S. Self‐hypnosis as antiemetic therapy in children receiving chemotherapy. Oncology Nursing Forum 1985;12(4):41‐6. - PubMed
Coyne 2006
-
- Coyne I. Children's experiences of hospitalization. Journal of Child Health Care: For Professionals Working with Children in the Hospital and Community 2006;10(4):326‐36. - PubMed
Culy 2001
-
- Culy CR, Bhana N, Plosker GL. Ondansetron: a review of its use as an antiemetic in children. Paediatric Drugs 2001;3(6):441‐79. - PubMed
Darbyshire 2005
-
- Darbyshire P, MacDougall C, Schiller W. Multiple methods in qualitative research with children: more insight or just more?. Qualitative Research 2005;5(4):417‐36.
Dodd 2001
-
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. Journal of Advanced Nursing 2001;33(5):668‐76. - PubMed
Dolgin 1985
-
- Dolgin MJ, Katz ER, McGinty K, Siegel SE. Anticipatory nausea and vomiting in pediatric cancer patients. Pediatrics 1985;75(3):547‐52. - PubMed
Dolgin 1989
-
- Dolgin MJ, Katz ER, Zeltzer LK, Landsverk J. Behavioral distress in pediatric patients with cancer receiving chemotherapy. Pediatrics 1989;84(1):103‐10. - PubMed
Dupuis 2006
-
- Dupuis LL, Taddio A, Kerr EN, Kelly A, MacKeigan L. Development and validation of the pediatric nausea assessment tool for use in children receiving antineoplastic agents. Pharmacotherapy 2006;26(9):1221‐31. - PubMed
Egger 1997
-
- Egger M, Zellweger‐Zohner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. The Lancet 1997;350(9074):326‐9. - PubMed
Einhorn 2005
-
- Einhorn LH, Rapoport B, Koeller J, Grunberg SM, Feyer P, Rittenberg C, et al. Antiemetic therapy for multiple‐day chemotherapy and high‐dose chemotherapy with stem cell transplant: review and consensus statement. Supportive Care in Cancer 2005;13(2):112‐6. - PubMed
Foot 1994
Gibson 2007
-
- Gibson F, Aldiss S, Kearney N, Miller M, Maguire R, McCann L, et al. An evaluation of an advanced symptom management system (ASyMS(C)) to monitor and manage chemotherapy related toxicity with young people. Centre for Research Nursing and Allied Health Professions, Great Ormond Street Hospital for Children and Cancer Care. London, 2007.
Glass 2002
-
- Glass N. UK charity to involve public in decision making for cancer research priorities. The Lancet 2002;360(9344):1487.
Hedstrom 2003
-
- Hedstrom M, Haglund K, Skolin I, Essen L. Distressing events for children and adolescents with cancer: child, parent, and nurse perceptions. Journal of Pediatric Oncology Nursing 2003;20(3):120‐32. - PubMed
Higgins 2009
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinds 2008
-
- Hinds PS. Patient‐reported outcomes: a desirable specialty standard for oncology or an incomplete measurement approach?. Cancer Nursing 2008;31(4):259‐60. - PubMed
Holdsworth 2006
-
- Holdsworth MT, Raisch DW, Frost J. Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer 2006;106(4):931‐40. - PubMed
Juni 2002
-
- Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta‐analyses of controlled trials: empirical study. International Journal of Epidemiology 2002;31(1):115‐23. - PubMed
Kremer 2014
-
- Kremer LCM, Leclercq E, Dalen EC. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2014, issue 8. Art. No.: CHILDCA.
Kris 2005
-
- Kris MG, Hesketh PJ, Herrstedt J, Rittenberg C, Einhorn LH, Grunberg SM, et al. Consensus proposals for the prevention of acute and delayed vomiting and nausea following high‐emetic‐risk chemotherapy. Supportive Care in Cancer 2005;13(2):85‐96. - PubMed
Lansky 1987
-
- Lansky SB, List MA, Lansky LL, Ritter‐Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer 1987;60:1651‐6. - PubMed
Linder 2005
-
- Linder LA. Measuring physical symptoms in children and adolescents with cancer. Cancer Nursing 2005;28(1):16‐26. - PubMed
McCorcle 1998
-
- McCorcle R, Cooley M, Shea JA. A User's Manual for the Symptom Distress Scale. Philadelphia: University of Pennsylvania ‐ National Institute of Nursing Research, 1998.
Meyer 2006
-
- Meyer S, Eden T, Kalirai H. Dexamethasone protects against cisplatin‐induced activation of the mitochondrial apoptotic pathway in human osteosarcoma cells. Cancer Biology & Therapy 2006;5(8):915‐20. - PubMed
Moher 2003
-
- Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technology Assessment (Winchester, England) 2003;7(41):1‐90. - PubMed
Moody 2006
-
- Moody K, Meyer M, Mancuso CA, Charlson M, Robbins L. Exploring concerns of children with cancer. Supportive Care in Cancer 2006;14(9):960‐6. - PubMed
Peters 2008
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Rheingans 2008
-
- Rheingans JI. Pediatric oncology nurses' management of patients' symptoms. Journal of Pediatric Oncology Nursing 2008;25(6):303‐11. - PubMed
Richardson 2007
-
- Richardson J, Smith JE, McCall G, Richardson A, Pilkington K, Kirsch I. Hypnosis for nausea and vomiting in cancer chemotherapy: a systematic review of the research evidence. European Journal of Cancer Care 2007;16(5):402‐12. - PubMed
Roila 2005
-
- Roila F, Feyer P, Maranzano E, Olver I, Clark‐Snow R, Warr D, et al. Antiemetics in children receiving chemotherapy. Supportive Care in Cancer 2005;13(2):129‐31. - PubMed
Sammons 2009
-
- Sammons H. Ethical issues of clinical trials in children: a European perspective. Archives of Disease in Childhood 2009;94(6):474‐7. - PubMed
Skoup 1990
-
- Skoup M. A comparison of two dose levels of granisetron in patients receiving high dose cisplatin. European Journal of Cancer 1990;26(Suppl 1):15‐9. - PubMed
Smith 1990
-
- Smith IE. A comparison of two dose levels of granisetron in patients receiving moderately emetogenic cytostatic chemotherapy. European Journal of Cancer 1990;26(Suppl 1):19‐23. - PubMed
Sutton 2008
-
- Sutton AJ, Higgins JPT. Recent developments in meta‐analysis. Statistics in Medicine 2008;27(5):625‐50. - PubMed
Tallon 2000
-
- Tallon D, Chard J, Dieppe P. Relation between agendas of the research community and the research consumer. The Lancet 2000;355(9220):2037‐40. - PubMed
Tremont‐Lukats 2007
-
- Tremont‐Lukats IW, Bruera E, González‐Barboteo J. Neurokinin‐1 receptor antagonists for prevention of chemotherapy‐related nausea and vomiting in adults. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006844] - DOI
WHO 2007
-
- WHO. Make medicines child size. http://www.who.int/childmedicines/en/index.html 6 Dec 2007 (accessed October 2009).
Wilby 2005
-
- Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al. Clinical effectiveness, tolerability and cost‐effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2005;9(15):1‐157, iii‐iv‐1‐157, iii‐iv. - PubMed
Williams 2006
-
- Williams PD, Schmideskamp J, Ridder EL, Williams AR. Symptom monitoring and dependent care during cancer treatment in children: pilot study. Cancer Nursing 2006;29(3):188‐97. - PubMed
Wolfe 2000
-
- Wolfe J, Grier HE, Klar N, Levin SB, Ellenbogen JM, Salem‐Schatz S, et al. Symptoms and suffering at the end of life in children with cancer. The New England Journal of Medicine 2000;342(5):326‐33. - PubMed
Woodgate 2003
-
- Woodgate RL, Degner LF, Yanofsky R. A different perspective to approaching cancer symptoms in children. Journal of Pain and Symptom Management 2003;26(3):800‐17. - PubMed
Woodgate 2005
-
- Woodgate RL. A different way of being: adolescents' experiences with cancer. Cancer Nursing 2005;28(1):8‐15. - PubMed
Zeltzer 1991
-
- Zeltzer LK, Dolgin MJ, LeBaron S, LeBaron C. A randomized, controlled study of behavioral intervention for chemotherapy distress in children with cancer. Pediatrics 1991;88(1):34‐42. - PubMed
Zhang 2006
-
- Zhang C, Beckermann B, Kallifatidis G, Liu Z, Rittgen W, Edler L, et al. Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. International Journal of Oncology 2006;29(5):1295‐301. - PubMed
Zou 2007
-
- Zou GY. One relative risk versus two odds ratios: implications for meta‐analyses involving paired and unpaired binary data. Clinical Trials 2007;4(1):25‐31. - PubMed
References to other published versions of this review
Phillips 2009
Phillips 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

