Toward Optimum Benefit-Risk and Reduced Access Lag For Cancer Drugs in Asia: A Global Development Framework Guided by Clinical Pharmacology Principles
- PMID: 26836226
- PMCID: PMC5351319
- DOI: 10.1111/cts.12386
Toward Optimum Benefit-Risk and Reduced Access Lag For Cancer Drugs in Asia: A Global Development Framework Guided by Clinical Pharmacology Principles
Figures
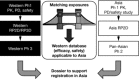
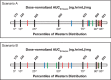
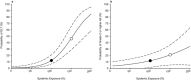
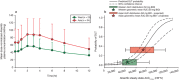
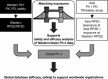
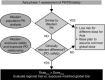

References
-
- Ferlay, J. et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015). - PubMed
-
- Bray, F. , Jemal, A. , Grey, N. , Ferlay, J. & Forman, D. Global cancer transitions according to the Human Development Index (2008‐2030): a population‐based study. Lancet Oncol. 13, 790–801 (2012). - PubMed
-
- Uyama, Y. , Shibata, T. , Nagai, N. , Hanaoka, H. , Toyoshima, S. & Mori, K. Successful bridging strategy based on ICH E5 guideline for drugs approved in Japan. Clin. Pharmacol. Ther. 78, 102–113 (2005). - PubMed
-
- Su, L.‐L. , Chern, H.‐D. , Ryan Lee, I.‐L. , Lin, C.‐L. & Lin, M.‐S. An overview of bridging study evaluation in Taiwan. Drug Inf. J. 43, 371–376 (2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

