Pregabalin for decreasing pancreatic pain in chronic pancreatitis
- PMID: 26836292
- PMCID: PMC8265874
- DOI: 10.1002/14651858.CD011522.pub2
Pregabalin for decreasing pancreatic pain in chronic pancreatitis
Abstract
Background: Chronic abdominal pain is one of the major symptoms in people with chronic pancreatitis. The role of pregabalin in people with chronic pancreatic pain due to chronic pancreatitis is uncertain.
Objectives: To assess the benefits and harms of pregabalin in people with chronic abdominal pain due to chronic pancreatitis.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library 2015, issue 6, and MEDLINE, EMBASE, Science Citation Index Expanded, trials registers until June 2015. We also searched the references of included trials to identify further trials.
Selection criteria: We considered only randomised controlled trials (RCT) performed in people with chronic pancreatic pain due to chronic pancreatitis, irrespective of language, blinding, or publication status for inclusion in the review.
Data collection and analysis: Two review authors independently identified trials and independently extracted data. We calculated the risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI) with RevMan 5, based on intention-to-treat analysis.
Main results: Only one study, funded by Pfizer, met the inclusion criteria for the review. A total of 64 participants (with chronic pain due to chronic pancreatitis) were randomly assigned to receive escalating doses of pregabalin (150 mg per day to 600 mg per day; 34 participants) or matching placebo (30 participants). Participants received pregabalin or placebo for three weeks on an outpatient basis; the outcomes were measured at the end of the treatment (i.e. three weeks from commencement of treatment). Potential participants taking concomitant analgesic medication and expected to stay on a stable regime during the trial were allowed to enter the study. This trial was at low risk of bias. The overall quality of evidence was low or moderate.Only the short-term outcomes were available in this trial. The medium and long-term outcomes, number of work days lost, and length of hospital stay due to admissions for pain control were not available. This trial found that the changes in opiate use (MD -26.00 mg; 95% CI -47.36 to -4.64; participants = 64; moderate-quality evidence), and pain score percentage changes from baseline (MD -12.00; 95% CI -21.82 to -2.18; participants = 64; moderate-quality evidence) were better in participants taking pregabalin compared to those taking placebo. This trial also found that there were more adverse events in participants taking pregabalin compared to those taking placebo (RR 1.71; 95% CI 1.20 to 2.43; participants = 64). The differences between pregabalin and placebo were imprecise for short-term health-related quality of life measured with the EORTC CLQ-30 questionnaire (MD 11.40; 95% CI -3.28 to 26.08; participants = 64; moderate-quality evidence), proportion of people with serious adverse events (RR 1.76; 95% CI 0.35 to 8.96; participants = 64; low-quality evidence), and proportion of people requiring hospital admissions (RR 0.44; 95% CI 0.04 to 4.62; participants = 64; low quality evidence).
Authors' conclusions: Based on low- to moderate-quality evidence, short-term use of pregabalin decreases short-term opiate use, and short-term pain scores, but increases the adverse events compared to placebo, in people with chronic pain due to chronic pancreatitis. The clinical implication of the decreases in short-term opiate use and short-term pain scores is not known.Future trials assessing the role of pregabalin in decreasing chronic pain in chronic pancreatitis should assess the medium- or long-term effects of pregabalin and should include outcomes such as, quality of life, treatment-related adverse events, number of work days lost, number of hospital admissions, and the length of hospital stay, in addition to pain scores, to assess the clinical and socioeconomic impact.
Conflict of interest statement
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
KG: none known.
CL: none known.
BRD: none known.
Figures
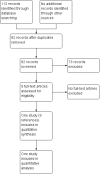

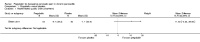
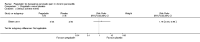




Update of
- doi: 10.1002/14651858.CD011522
References
References to studies included in this review
Olesen 2011 {published data only}
-
- Bouwense SAW, Olesen SS, Drewes AM, Poley JW, Goor H, Wilder‐Smith OHG. Pregabalin reduces central sensitisation in chronic pancreatitis. European Journal of Pain Supplements 2011;5(1):21.
-
- Graversen C, Olesen SS, Olesen AE, Drewes AM. The analgesic effect of pregabalin is correlated to alterations in pharmaco‐EEG in chronic pain patients. European Journal of Pain Supplements 2011;5(1):113.
-
- Malver LP, Olesen SS, Graversen C, Olesen AE, Frokjaer JB, Wilder‐Smith O, et al. Effect of pregabalin on visceral sensation and central pain processing in patients with chronic pancreatitis. European Journal of Pain Supplements 2011;5(1):277.
Additional references
Ahmed Ali 2013
Ammann 1997
-
- Ammann RW. A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas 1997;14(3):215‐21. - PubMed
Bagul 2006
-
- Bagul A, Siriwardena AK. Evaluation of the Manchester classification system for chronic pancreatitis. Journal of the Pancreas 2006;7(4):390‐6. - PubMed
Bradley 2003
-
- Bradley 3rd EL, Bem J. Nerve blocks and neuroablative surgery for chronic pancreatitis. World Journal of Surgery 2003;27(11):1241‐8. - PubMed
Braganza 2011
-
- Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet 2011;377(9772):1184‐97. - PubMed
Buchler 2009
Demets 1987
-
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dite 2001
-
- Dite P, Stary K, Novotny I, Precechtelova M, Dolina J, Lata J, et al. Incidence of chronic pancreatitis in the Czech Republic. European Journal of Gastroenterology and Hepatology 2001;13(6):749‐50. - PubMed
Dominguez‐Munoz 2014
-
- Dominguez‐Munoz JE, Lucendo A, Carballo LF, Iglesias‐Garcia J, Tenias JM. A Spanish multicenter study to estimate the prevalence and incidence of chronic pancreatitis and its complications. Revista Española de Enfermedades Digestivas 2014;106(4):239‐45. - PubMed
Egger 1997
EuroQol 2014
-
- EuroQol. About EQ‐5D. http://www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
Fasanella 2007
-
- Fasanella KE, Davis B, Lyons J, Chen Z, Lee KK, Slivka A, et al. Pain in chronic pancreatitis and pancreatic cancer. Gastroenterology Clinics of North America 2007;36(2):335‐64, ix. - PubMed
FDA 2006
-
- Center for Biologics Evaluation and Research, U.S. Food, Drug Administration. Guidance for industry adverse reactions section of labelling for human prescription drug and biological products — Content and format. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... 2006 (accessed 4 July 2014).
Gajraj 2007
-
- Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesthesia and Analgesia 2007;105(6):1805‐15. - PubMed
Hall 2014
-
- Hall TC, Garcea G, Webb MA, Al‐Leswas D, Metcalfe MS, Dennison AR. The socio‐economic impact of chronic pancreatitis: a systematic review. Journal of Evaluation in Clinical Practice 2014;20(3):203‐7. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1996
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996.
Joergensen 2010
-
- Joergensen M, Brusgaard K, Cruger DG, Gerdes AM, Muckadell OB. Incidence, prevalence, etiology, and prognosis of first‐time chronic pancreatitis in young patients: a nationwide cohort study. Digestive Diseases and Sciences 2010;55(10):2988‐98. - PubMed
Kanbayashi 2014
-
- Kanbayashi Y, Onishi K, Hosokawa T. Factors predicting adverse events associated with pregabalin administered for neuropathic pain relief. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur 2014;19(6):e164‐7. - PMC - PubMed
Martindale 2011
-
- Sweetman S (editor). Martindale: the complete drug reference (online version), 37th edition. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference 2011 (accessed 23 September 2014).
MHRA 2013
-
- Medicines and Healthcare products Regulatory Agency (MHRA). Clinical trials for medicines: Safety reporting ‐ SUSARs and DSURs. http://www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clin... 2013 (accessed 4 July 2014).
NCBI 2014
-
- NCBI. MeSH. NLM Controlled Vocabulary. Pancreas. http://www.ncbi.nlm.nih.gov/mesh/68010179 2014 (accessed 4 July 2014).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Puli 2009
-
- Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS‐guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta‐analysis and systematic review. Digestive Diseases and Sciences 2009;54(11):2330‐7. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schneider 2007
-
- Schneider A, Lohr JM, Singer MV. The M‐ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. Journal of Gastroenterology 2007;42(2):101‐19. - PubMed
Shimosegawa 2010
-
- Shimosegawa T, Kataoka K, Kamisawa T, Miyakawa H, Ohara H, Ito T, et al. The revised Japanese clinical diagnostic criteria for chronic pancreatitis. Journal of Gastroenterology 2010;45(6):584‐91. - PubMed
Sills 2006
-
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current Opinion in Pharmacology 2006;6(1):108‐13. - PubMed
Spanier 2013
Ware 2014
-
- Ware JE. SF‐36® Health Survey Update. http://www.sf‐36.org/tools/sf36.shtml 2014 (accessed 8 October 2014).
Yadav 2011
-
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population‐based study. American Journal of Gastroenterology 2011;106(12):2192‐9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

