Exercise leads to the re-emergence of the cholinergic/nestin neuronal phenotype within the medial septum/diagonal band and subsequent rescue of both hippocampal ACh efflux and spatial behavior
- PMID: 26836322
- PMCID: PMC4794758
- DOI: 10.1016/j.expneurol.2016.01.018
Exercise leads to the re-emergence of the cholinergic/nestin neuronal phenotype within the medial septum/diagonal band and subsequent rescue of both hippocampal ACh efflux and spatial behavior
Abstract
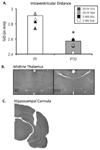
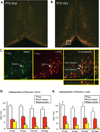
Exercise has been shown to improve cognitive functioning in a range of species, presumably through an increase in neurotrophins throughout the brain, but in particular the hippocampus. The current study assessed the ability of exercise to restore septohippocampal cholinergic functioning in the pyrithiamine-induced thiamine deficiency (PTD) rat model of the amnestic disorder Korsakoff Syndrome. After voluntary wheel running or sedentary control conditions (stationary wheel attached to the home cage), PTD and control rats were behaviorally tested with concurrent in vivo microdialysis, at one of two time points: 24-h or 2-weeks post-exercise. It was found that only after the 2-week adaption period did exercise lead to an interrelated sequence of events in PTD rats that included: (1) restored spatial working memory; (2) rescued behaviorally-stimulated hippocampal acetylcholine efflux; and (3) within the medial septum/diagonal band, the re-emergence of the cholinergic (choline acetyltransferase [ChAT+]) phenotype, with the greatest change occurring in the ChAT+/nestin+ neurons. Furthermore, in control rats, exercise followed by a 2-week adaption period improved hippocampal acetylcholine efflux and increased the number of neurons co-expressing the ChAT and nestin phenotype. These findings demonstrate a novel mechanism by which exercise can modulate the mature cholinergic/nestin neuronal phenotype leading to improved neurotransmitter function as well as enhanced learning and memory.
Keywords: Acetylcholine; BDNF; Diagonal band; Exercise; Medial Septum; NGF; Nestin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Selective septohippocampal - but not forebrain amygdalar - cholinergic dysfunction in diencephalic amnesia.Brain Res. 2007 Mar 30;1139:210-9. doi: 10.1016/j.brainres.2006.12.083. Epub 2007 Jan 8. Brain Res. 2007. PMID: 17289001 Free PMC article.
-
The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia.Neuroscience. 2009 Apr 21;160(1):32-41. doi: 10.1016/j.neuroscience.2009.02.044. Epub 2009 Mar 3. Neuroscience. 2009. PMID: 19264109 Free PMC article.
-
Differential cortical neurotrophin and cytogenetic adaptation after voluntary exercise in normal and amnestic rats.Neuroscience. 2014 Jan 31;258:131-46. doi: 10.1016/j.neuroscience.2013.10.075. Epub 2013 Nov 9. Neuroscience. 2014. PMID: 24215977 Free PMC article.
-
Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery.Neuropsychol Rev. 2012 Jun;22(2):195-209. doi: 10.1007/s11065-012-9194-1. Epub 2012 Apr 13. Neuropsychol Rev. 2012. PMID: 22528861 Free PMC article. Review.
-
Behavioral and neurochemical alterations following thiamine deficiency in rodents: relationship to functions of cholinergic neurons.Yakugaku Zasshi. 2005 Jul;125(7):549-54. doi: 10.1248/yakushi.125.549. Yakugaku Zasshi. 2005. PMID: 15997211 Review.
Cited by
-
Interaction of aerobic exercise and crocin improves memory, learning and hypocampic tau and neurotrophins gene expression in rats treated with trimethytin as a model of Alzheimer's disease.Mol Biol Rep. 2024 Jan 16;51(1):111. doi: 10.1007/s11033-023-09197-4. Mol Biol Rep. 2024. PMID: 38227208
-
Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation.Sci Rep. 2017 Jan 19;7:40802. doi: 10.1038/srep40802. Sci Rep. 2017. PMID: 28102288 Free PMC article.
-
Nerve Growth Factor Is Responsible for Exercise-Induced Recovery of Septohippocampal Cholinergic Structure and Function.Front Neurosci. 2018 Nov 1;12:773. doi: 10.3389/fnins.2018.00773. eCollection 2018. Front Neurosci. 2018. PMID: 30443202 Free PMC article.
-
Understanding How Physical Exercise Improves Alzheimer's Disease: Cholinergic and Monoaminergic Systems.Front Aging Neurosci. 2022 May 18;14:869507. doi: 10.3389/fnagi.2022.869507. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35663578 Free PMC article. Review.
-
Examination of cortically projecting cholinergic neurons following exercise and environmental intervention in a rodent model of fetal alcohol spectrum disorders.Birth Defects Res. 2021 Feb 1;113(3):299-313. doi: 10.1002/bdr2.1839. Epub 2020 Nov 10. Birth Defects Res. 2021. PMID: 33174398 Free PMC article.
References
-
- Anzalone S, Vetreno RP, Ramos RL, Savage LM. Cortical cholinergic abnormalities contribute to the amnesic state induced by pyrithiamine-induced thiamine deficiency in the rat. European Journal of Neuroscience. 2010;32(5):847–858. http://doi.org/10.1111/j.1460-9568.2010.07358.x. - DOI - PMC - PubMed
-
- Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167(3):588–597. http://doi.org/10.1016/j.neuroscience.2010.02.050. - DOI - PMC - PubMed
-
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nature Protocols. 2006;1(3):1306–1311. http://doi.org/10.1038/nprot.2006.205. - DOI - PubMed
-
- Burke MA, Mobley WC, Cho J, Wiegand SJ, Lindsay RM, Mufson EJ, Kordower JH. Loss of developing cholinergic basal forebrain neurons following excitotoxic lesions of the hippocampus: rescue by neurotrophins. Experimental Neurology. 1994;130(2):178–195. http://doi.org/10.1006/exnr.1994.1197. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

