Duality at the gate: Skin dendritic cells as mediators of vaccine immunity and tolerance
- PMID: 26836327
- PMCID: PMC4962728
- DOI: 10.1080/21645515.2015.1066050
Duality at the gate: Skin dendritic cells as mediators of vaccine immunity and tolerance
Abstract
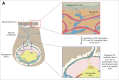
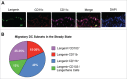
Since Edward Jenner's discovery that intentional exposure to cowpox could provide lifelong protection from smallpox, vaccinations have been a major focus of medical research. However, while the protective benefits of many vaccines have been successfully translated into the clinic, the cellular and molecular mechanisms that differentiate effective vaccines from sub-optimal ones are not well understood. Dendritic cells (DCs) are the gatekeepers of the immune system, and are ultimately responsible for the generation of adaptive immunity and lifelong protective memory through interactions with T cells. In addition to lymph node and spleen resident DCs, a number of tissue resident DC populations have been identified at barrier tissues, such as the skin, which migrate to the local lymph node (migDC). These populations have unique characteristics, and play a key role in the function of cutaneous vaccinations by shuttling antigen from the vaccination site to the draining lymph node, rapidly capturing freely draining antigens in the lymph node, and providing key stimuli to T cells. However, while migDCs are responsible for the generation of immunity following exposure to certain pathogens and vaccines, recent work has identified a tolerogenic role for migDCs in the steady state as well as during protein immunization. Here, we examine the roles and functions of skin DC populations in the generation of protective immunity, as well as their role as regulators of the immune system.
Keywords: DC targeted vaccines; dendritic cells; immune tolerance; migratory DCs.
Figures
References
-
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973; 137:1142-62; PMID:4573839; http://dx.doi.org/10.1084/jem.137.5.1142 - DOI - PMC - PubMed
-
- Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, et al.. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol 2003; 4:380-6; PMID:12598895; http://dx.doi.org/10.1038/ni903 - DOI - PubMed
-
- Anderson KL, Perkin H, Surh CD, Venturini S, Maki RA, Torbett BE. Transcription factor PU.1 is necessary for development of thymic and myeloid progenitor-derived dendritic cells. J Immunol 2000; 164:1855-61; PMID:10657634; http://dx.doi.org/10.4049/jimmunol.164.4.1855 - DOI - PubMed
-
- Guerriero A, Langmuir PB, Spain LM, Scott EW. PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood 2000; 95:879-85; PMID:10648399 - PubMed
-
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, et al.. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000; 95:3489-97; PMID:10828034 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
