Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4
- PMID: 26837267
- PMCID: PMC4742841
- DOI: 10.1038/ncomms10521
Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4
Erratum in
-
Corrigendum: Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4.Nat Commun. 2016 Jun 7;7:11907. doi: 10.1038/ncomms11907. Nat Commun. 2016. PMID: 27271082 Free PMC article. No abstract available.
Abstract
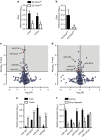
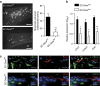
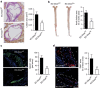
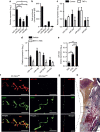
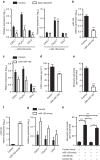
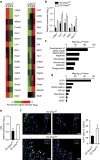
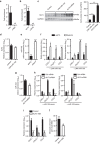
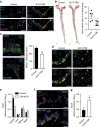
MicroRNAs regulate the maladaptation of endothelial cells (ECs) to naturally occurring disturbed blood flow at arterial bifurcations resulting in arterial inflammation and atherosclerosis in response to hyperlipidemic stress. Here, we show that reduced endothelial expression of the RNAse Dicer, which generates almost all mature miRNAs, decreases monocyte adhesion, endothelial C-X-C motif chemokine 1 (CXCL1) expression, atherosclerosis and the lesional macrophage content in apolipoprotein E knockout mice (Apoe(-/-)) after exposure to a high-fat diet. Endothelial Dicer deficiency reduces the expression of unstable miRNAs, such as miR-103, and promotes Krüppel-like factor 4 (KLF4)-dependent gene expression in murine atherosclerotic arteries. MiR-103 mediated suppression of KLF4 increases monocyte adhesion to ECs by enhancing nuclear factor-κB-dependent CXCL1 expression. Inhibiting the interaction between miR-103 and KLF4 reduces atherosclerosis, lesional macrophage accumulation and endothelial CXCL1 expression. Overall, our study suggests that Dicer promotes endothelial maladaptation and atherosclerosis in part by miR-103-mediated suppression of KLF4.
Figures
Similar articles
-
Endothelial Hypoxia-Inducible Factor-1α Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a.Hypertension. 2015 Dec;66(6):1220-6. doi: 10.1161/HYPERTENSIONAHA.115.05886. Epub 2015 Oct 19. Hypertension. 2015. PMID: 26483345
-
The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis.Circulation. 2013 Apr 16;127(15):1609-19. doi: 10.1161/CIRCULATIONAHA.112.000736. Epub 2013 Mar 19. Circulation. 2013. PMID: 23513069
-
Dicer in Macrophages Prevents Atherosclerosis by Promoting Mitochondrial Oxidative Metabolism.Circulation. 2018 Oct 30;138(18):2007-2020. doi: 10.1161/CIRCULATIONAHA.117.031589. Circulation. 2018. PMID: 29748186
-
Mechanisms of MicroRNAs in Atherosclerosis.Annu Rev Pathol. 2016 May 23;11:583-616. doi: 10.1146/annurev-pathol-012615-044135. Annu Rev Pathol. 2016. PMID: 27193456 Review.
-
Chemokines and microRNAs in atherosclerosis.Cell Mol Life Sci. 2015 Sep;72(17):3253-66. doi: 10.1007/s00018-015-1925-z. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001902 Free PMC article. Review.
Cited by
-
Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease.J Clin Med. 2022 Nov 20;11(22):6849. doi: 10.3390/jcm11226849. J Clin Med. 2022. PMID: 36431326 Free PMC article. Review.
-
LINC00460 Stimulates the Proliferation of Vascular Endothelial Cells by Downregulating miRNA-24-3p.Dis Markers. 2022 Feb 18;2022:2524156. doi: 10.1155/2022/2524156. eCollection 2022. Dis Markers. 2022. PMID: 35222741 Free PMC article.
-
circARF3 Alleviates Mitophagy-Mediated Inflammation by Targeting miR-103/TRAF3 in Mouse Adipose Tissue.Mol Ther Nucleic Acids. 2019 Mar 1;14:192-203. doi: 10.1016/j.omtn.2018.11.014. Epub 2018 Dec 1. Mol Ther Nucleic Acids. 2019. PMID: 30623853 Free PMC article.
-
Inflammatory microRNAs in cardiovascular pathology: another brick in the wall.Front Immunol. 2023 May 18;14:1196104. doi: 10.3389/fimmu.2023.1196104. eCollection 2023. Front Immunol. 2023. PMID: 37275892 Free PMC article. Review.
-
MicroRNAs Modulate Oxidative Stress in Hypertension through PARP-1 Regulation.Oxid Med Cell Longev. 2017;2017:3984280. doi: 10.1155/2017/3984280. Epub 2017 Jun 4. Oxid Med Cell Longev. 2017. PMID: 28660007 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

