A Hypersweet Protein: Removal of The Specific Negative Charge at Asp21 Enhances Thaumatin Sweetness
- PMID: 26837600
- PMCID: PMC4738316
- DOI: 10.1038/srep20255
A Hypersweet Protein: Removal of The Specific Negative Charge at Asp21 Enhances Thaumatin Sweetness
Abstract
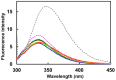
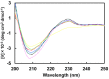
Thaumatin is an intensely sweet-tasting protein that elicits sweet taste at a concentration of 50 nM, a value 100,000 times larger than that of sucrose on a molar basis. Here we attempted to produce a protein with enhanced sweetness by removing negative charges on the interacting side of thaumatin with the taste receptor. We obtained a D21N mutant which, with a threshold value 31 nM is much sweeter than wild type thaumatin and, together with the Y65R mutant of single chain monellin, one of the two sweetest proteins known so far. The complex model between the T1R2-T1R3 sweet receptor and thaumatin, derived from tethered docking in the framework of the wedge model, confirmed that each of the positively charged residues critical for sweetness is close to a receptor residue of opposite charge to yield optimal electrostatic interaction. Furthermore, the distance between D21 and its possible counterpart D433 (located on the T1R2 protomer of the receptor) is safely large to avoid electrostatic repulsion but, at the same time, amenable to a closer approach if D21 is mutated into the corresponding asparagine. These findings clearly confirm the importance of electrostatic potentials in the interaction of thaumatin with the sweet receptor.
Figures



Similar articles
-
Subatomic structure of hyper-sweet thaumatin D21N mutant reveals the importance of flexible conformations for enhanced sweetness.Biochimie. 2019 Feb;157:57-63. doi: 10.1016/j.biochi.2018.10.020. Epub 2018 Oct 31. Biochimie. 2019. PMID: 30389513
-
The Flexible Loop is a New Sweetness Determinant Site of the Sweet-Tasting Protein: Characterization of Novel Sweeter Mutants of the Single-Chain Monellin (MNEI).Chem Senses. 2019 Oct 17;44(8):607-614. doi: 10.1093/chemse/bjz057. Chem Senses. 2019. PMID: 31504288
-
Critical molecular regions for elicitation of the sweetness of the sweet-tasting protein, thaumatin I.FEBS J. 2008 Jul;275(14):3644-52. doi: 10.1111/j.1742-4658.2008.06509.x. FEBS J. 2008. PMID: 18544096
-
[Advances in genetic engineering and molecular modification of sweet-tasting proteins].Sheng Wu Gong Cheng Xue Bao. 2025 Feb 25;41(2):559-573. doi: 10.13345/j.cjb.240123. Sheng Wu Gong Cheng Xue Bao. 2025. PMID: 39989056 Review. Chinese.
-
Hypothesis/review: the structural basis of sweetness perception of sweet-tasting plant proteins can be deduced from sequence analysis.Plant Sci. 2011 Oct;181(4):347-54. doi: 10.1016/j.plantsci.2011.06.009. Epub 2011 Jun 23. Plant Sci. 2011. PMID: 21889040 Review.
Cited by
-
Metabolic Effects of the Sweet Protein MNEI as a Sweetener in Drinking Water. A Pilot Study of a High Fat Dietary Regimen in a Rodent Model.Nutrients. 2019 Nov 4;11(11):2643. doi: 10.3390/nu11112643. Nutrients. 2019. PMID: 31689911 Free PMC article.
-
Transcriptome and miRNA analyses of the response to Corynespora cassiicola in cucumber.Sci Rep. 2018 May 17;8(1):7798. doi: 10.1038/s41598-018-26080-6. Sci Rep. 2018. PMID: 29773833 Free PMC article.
-
High-level production of single chain monellin mutants with enhanced sweetness and stability in tobacco chloroplasts.Planta. 2018 Aug;248(2):465-476. doi: 10.1007/s00425-018-2920-z. Epub 2018 May 18. Planta. 2018. PMID: 29777363
-
Positive Charges on the Surface of Thaumatin Are Crucial for the Multi-Point Interaction with the Sweet Receptor.Front Mol Biosci. 2018 Feb 13;5:10. doi: 10.3389/fmolb.2018.00010. eCollection 2018. Front Mol Biosci. 2018. PMID: 29487853 Free PMC article.
-
Sweeter and stronger: enhancing sweetness and stability of the single chain monellin MNEI through molecular design.Sci Rep. 2016 Sep 23;6:34045. doi: 10.1038/srep34045. Sci Rep. 2016. PMID: 27658853 Free PMC article.
References
-
- van der Wel H. & Loeve K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur. J. Biochem. 31, 221–225 (1972). - PubMed
-
- Iyengar R. B. et al. The complete amino-acid sequence of the sweet protein thaumatin I. Eur. J. Biochem. 96, 193–204 (1979). - PubMed
-
- Edens L. et al. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene 18, 1–12 (1982). - PubMed
-
- Ide N., Masuda T. & Kitabatake N. Effects of pre- and pro-sequence of thaumatin on the secretion by Pichia pastoris. Biochem. Biophys. Res. Commun. 363, 708–714 (2007). - PubMed
-
- Kaneko R. & Kitabatake N. Structure-sweetness relationship study of sweet protein thaumatin: Importance of lysine residues. Chem. Senses 26, 167–177 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

