Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs
- PMID: 26837601
- PMCID: PMC4738272
- DOI: 10.1038/srep20030
Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs
Abstract
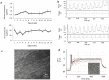
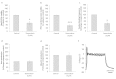
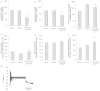
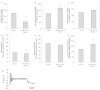
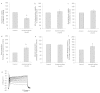
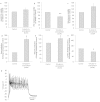
We report on a functional human model to evaluate multi-organ toxicity in a 4-organ system under continuous flow conditions in a serum-free defined medium utilizing a pumpless platform for 14 days. Computer simulations of the platform established flow rates and resultant shear stress within accepted ranges. Viability of the system was demonstrated for 14 days as well as functional activity of cardiac, muscle, neuronal and liver modules. The pharmacological relevance of the integrated modules were evaluated for their response at 7 days to 5 drugs with known side effects after a 48 hour drug treatment regime. The results of all drug treatments were in general agreement with published toxicity results from human and animal data. The presented phenotypic culture model exhibits a multi-organ toxicity response, representing the next generation of in vitro systems, and constitutes a step towards an in vitro "human-on-a-chip" assay for systemic toxicity screening.
Conflict of interest statement
JJH and MLS have potential competing financial interests, in that a company has been formed to market services for the type of device described herein in which they have a financial interest.
Figures


References
-
- Wysowski D. K. & Swartz L. Adverse drug event surveillance and drug withdrawals in the united states, 1969–2002: The importance of reporting suspected reactions. Arch. Intern. Med. 165, 1363–1369 (2005). - PubMed
-
- Hay M., Thomas D. W., Craighead J. L., Economides C. & Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotech. 32, 40–51 (2014). - PubMed
-
- Huh D., Torisawa Y. S., Hamilton G. A., Kim H. J. & Ingber D. E. Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12, 2156–2164 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

