Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial
- PMID: 26837842
- PMCID: PMC4858585
- DOI: 10.1038/leu.2016.5
Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial
Abstract
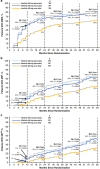
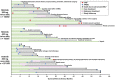
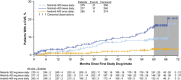
In the phase 3 Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients (ENESTnd) study, nilotinib resulted in earlier and higher response rates and a lower risk of progression to accelerated phase/blast crisis (AP/BC) than imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP). Here, patients' long-term outcomes in ENESTnd are evaluated after a minimum follow-up of 5 years. By 5 years, more than half of all patients in each nilotinib arm (300 mg twice daily, 54%; 400 mg twice daily, 52%) achieved a molecular response 4.5 (MR(4.5); BCR-ABL⩽0.0032% on the International Scale) compared with 31% of patients in the imatinib arm. A benefit of nilotinib was observed across all Sokal risk groups. Overall, safety results remained consistent with those from previous reports. Numerically more cardiovascular events (CVEs) occurred in patients receiving nilotinib vs imatinib, and elevations in blood cholesterol and glucose levels were also more frequent with nilotinib. In contrast to the high mortality rate associated with CML progression, few deaths in any arm were associated with CVEs, infections or pulmonary diseases. These long-term results support the positive benefit-risk profile of frontline nilotinib 300 mg twice daily in patients with CML-CP.
Conflict of interest statement
Authors declare the following relationships with pharmaceutical companies: Novartis—receipt of honoraria (AH, GS, TPH, DWK, PdlC, GE, REC, HN), research funding (all authors), nonfinancial support (GE, HN), employment (BD, WD, DD, HDM) and stock ownership (BD, DD, HDM); Pfizer—receipt of honoraria (AH, RAL, PdlC, GE, REC) and research funding (AH, DWK, REC); Ariad—receipt of honoraria (AH, TPH, GS, PdlC, GE) and research funding (AH, TPH); Bristol-Myers Squibb—receipt of honoraria (AH, GS, TPH, DWK, PdlC), research funding (AH, TPH, REC, IWF) and nonfinancial support (GE); Sanofi—receipt of honoraria (REC) and research funding (REC); Ilyang—receipt of honoraria (DWK).
Figures


References
-
- Tasigna (nilotinib) [package insert]. Novartis Pharmaceuticals Corporation: East Hanover, NJ, USA, 2015.
-
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259. - PubMed
-
- Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 2011; 12: 841–851. - PubMed
-
- Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia 2012; 26: 2197–2203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

