Aquaporin-5: from structure to function and dysfunction in cancer
- PMID: 26837927
- PMCID: PMC11108570
- DOI: 10.1007/s00018-016-2142-0
Aquaporin-5: from structure to function and dysfunction in cancer
Abstract
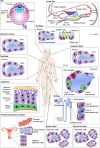
Aquaporins, a highly conserved group of membrane proteins, are involved in the bidirectional transfer of water and small solutes across cell membranes taking part in many biological functions all over the human body. In view of the wide range of cancer malignancies in which aquaporin-5 (AQP5) has been detected, an increasing interest in its implication in carcinogenesis has emerged. Recent publications suggest that this isoform may enhance cancer cell proliferation, migration and survival in a variety of malignancies, with strong evidences pointing to AQP5 as a promising drug target and as a novel biomarker for cancer aggressiveness with high translational potential for therapeutics and diagnostics. This review addresses the structural and functional features of AQP5, detailing its tissue distribution and functions in human body, its expression pattern in a variety of tumors, and highlighting the underlying mechanisms involved in carcinogenesis. Finally, the actual progress of AQP5 research, implications in cancer biology and potential for cancer detection and prognosis are discussed.
Keywords: Aquaporin; Biomarker; Permeability; Signaling pathways; Tumor; Water channel.
Figures




References
-
- Smith BL, Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991;266(10):6407–6415. - PubMed
-
- Agre P, Saboori AM, Asimos A, Smith BL. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem. 1987;262(36):17497–17503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

