Strigolactones: new plant hormones in action
- PMID: 26838034
- PMCID: PMC4875949
- DOI: 10.1007/s00425-015-2455-5
Strigolactones: new plant hormones in action
Abstract
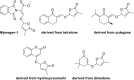
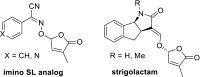
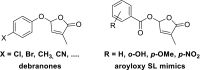
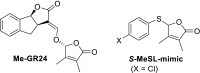
The key step in the mode of action of strigolactones is the enzymatic detachment of the D-ring. The thus formed hydroxy butenolide induces conformational changes of the receptor pocket which trigger a cascade of reactions in the signal transduction. Strigolactones (SLs) constitute a new class of plant hormones which are of increasing importance in plant science. For the last 60 years, they have been known as germination stimulants for parasitic plants. Recently, several new bio-properties of SLs have been discovered such as the branching factor for arbuscular mycorrhizal fungi, regulation of plant architecture (inhibition of bud outgrowth and of shoot branching) and the response to abiotic factors, etc. To broaden horizons and encourage new ideas for identifying and synthesising new and structurally simple SLs, this review is focused on molecular aspects of this new class of plant hormones. Special attention has been given to structural features, the mode of action of these phytohormones in various biological actions, the design of SL analogs and their applications.
Keywords: Karrikins; Mode of action; Signal transduction; Strigolactone analogs; Strigolactone mimics; Strigolactones.
Figures



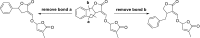





Similar articles
-
Structure and activity of strigolactones: new plant hormones with a rich future.Mol Plant. 2013 Jan;6(1):38-62. doi: 10.1093/mp/sss141. Epub 2012 Nov 30. Mol Plant. 2013. PMID: 23204499 Review.
-
Strigolactones: new plant hormones in the spotlight.J Exp Bot. 2018 Apr 23;69(9):2205-2218. doi: 10.1093/jxb/erx487. J Exp Bot. 2018. PMID: 29385517 Review.
-
New strigolactone mimics: structure-activity relationship and mode of action as germinating stimulants for parasitic weeds.Bioorg Med Chem Lett. 2013 Sep 15;23(18):5182-6. doi: 10.1016/j.bmcl.2013.07.004. Epub 2013 Jul 13. Bioorg Med Chem Lett. 2013. PMID: 23920440
-
Strigolactones: structures and biological activities.Pest Manag Sci. 2009 May;65(5):467-70. doi: 10.1002/ps.1726. Pest Manag Sci. 2009. PMID: 19222028 Review.
-
Structural diversity in the strigolactones.J Exp Bot. 2018 Apr 23;69(9):2219-2230. doi: 10.1093/jxb/ery091. J Exp Bot. 2018. PMID: 29522118 Review.
Cited by
-
Molecular basis of strigolactone perception in root-parasitic plants: aiming to control its germination with strigolactone agonists/antagonists.Cell Mol Life Sci. 2020 Mar;77(6):1103-1113. doi: 10.1007/s00018-019-03318-8. Epub 2019 Oct 5. Cell Mol Life Sci. 2020. PMID: 31587093 Free PMC article. Review.
-
Quantification of karrikins in smoke water using ultra-high performance liquid chromatography-tandem mass spectrometry.Plant Methods. 2019 Jul 25;15:81. doi: 10.1186/s13007-019-0467-z. eCollection 2019. Plant Methods. 2019. PMID: 31372177 Free PMC article.
-
Tailoring plant-associated microbial inoculants in agriculture: a roadmap for successful application.J Exp Bot. 2020 Jun 26;71(13):3878-3901. doi: 10.1093/jxb/eraa111. J Exp Bot. 2020. PMID: 32157287 Free PMC article. Review.
-
Network pharmacological evaluation of strigolactones efficacy as potential inhibitors against therapeutic targets of hepatocellular carcinoma.Biotechnol Lett. 2022 Jul;44(7):879-900. doi: 10.1007/s10529-022-03266-7. Epub 2022 Jun 7. Biotechnol Lett. 2022. PMID: 35672528
-
Orchids and their mycorrhizal fungi: an insufficiently explored relationship.Mycorrhiza. 2020 Jan;30(1):5-22. doi: 10.1007/s00572-020-00934-2. Epub 2020 Jan 25. Mycorrhiza. 2020. PMID: 31982950 Review.
References
-
- Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, Greb T. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA. 2011;108:20242–20247. doi: 10.1073/pnas.1111902108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

