PPARα-dependent cholesterol/testosterone disruption in Leydig cells mediates 2,4-dichlorophenoxyacetic acid-induced testicular toxicity in mice
- PMID: 26838045
- PMCID: PMC6334304
- DOI: 10.1007/s00204-016-1669-z
PPARα-dependent cholesterol/testosterone disruption in Leydig cells mediates 2,4-dichlorophenoxyacetic acid-induced testicular toxicity in mice
Abstract
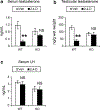
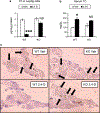
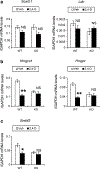
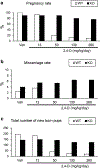
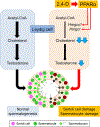
It was reported that 2,4-dichlorophenoxyacetic acid (2,4-D), a commonly used herbicide and a possible endocrine disruptor, can disturb spermatogenesis, but the precise mechanism is not understood. Since 2,4-D is a weak peroxisome proliferator in hepatocytes and peroxisome proliferator-activated receptor α (PPARα) is also expressed in Leydig cells, this study aimed to investigate the link between PPARα and 2,4-D-mediated testicular dysfunction. 2,4-D (130 mg/kg/day) was administered to wild-type and Ppara-null mice for 2 weeks, and the alterations in testis and testosterone/cholesterol metabolism in Leydig cells were examined. Treatment with 2,4-D markedly decreased testicular testosterone in wild-type mice, leading to degeneration of spermatocytes and Sertoli cells. The 2,4-D decreased cholesterol levels in Leydig cells of wild-type mice through down-regulating the expression of 3-hydroxy-3-methylglutaryl coenzyme A synthase 1 and reductase, involved in de novo cholesterogenesis. However, the mRNAs encoding the important proteins involved in testosterone synthesis were unchanged by 2,4-D except for CYP17A1, indicating that exhausted cholesterol levels in the cells is a main reason for reduced testicular testosterone. Additionally, pregnancy rate and the number of pups between 2,4-D-treated wild-type male mice and untreated female mice were significantly lower compared with those between untreated couples. These phenomena were not observed in 2,4-D-treated Ppara-null males. Collectively, these results suggest a critical role for PPARα in 2,4-D-induced testicular toxicity due to disruption of cholesterol/testosterone homeostasis in Leydig cells. This study yields novel insights into the possible mechanism of testicular dysfunction and male infertility caused by 2,4-D.
Keywords: 2,4-dichlorophenoxyacetic acid; Cholesterol; Leydig cell; PPARα; Testicular toxicity; Testosterone.
Figures



Similar articles
-
Disruption of rat testis development following combined in utero exposure to the phytoestrogen genistein and antiandrogenic plasticizer di-(2-ethylhexyl) phthalate.Biol Reprod. 2014 Sep;91(3):64. doi: 10.1095/biolreprod.114.120907. Epub 2014 Jul 16. Biol Reprod. 2014. PMID: 25031359
-
Disruption of spermatogenesis in testicular adult Wistar rats after short-term exposure to high dose of glyphosate based-herbicide: Histopathological and biochemical changes.Reprod Biol. 2024 Jun;24(2):100865. doi: 10.1016/j.repbio.2024.100865. Epub 2024 Feb 24. Reprod Biol. 2024. PMID: 38402720
-
Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARα.Toxicol Lett. 2011 Sep 10;205(3):265-72. doi: 10.1016/j.toxlet.2011.06.015. Epub 2011 Jun 25. Toxicol Lett. 2011. PMID: 21712084 Free PMC article.
-
The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: An update.Syst Biol Reprod Med. 2018 Apr;64(2):93-102. doi: 10.1080/19396368.2017.1422046. Epub 2018 Jan 4. Syst Biol Reprod Med. 2018. PMID: 29299971 Review.
-
Leydig cells.Contraception. 1975 May;11(5):571-606. doi: 10.1016/0010-7824(75)90111-0. Contraception. 1975. PMID: 1095296 Review.
Cited by
-
Effect of Pesticides on Peroxisome Proliferator-Activated Receptors (PPARs) and Their Association with Obesity and Diabetes.PPAR Res. 2023 Feb 24;2023:1743289. doi: 10.1155/2023/1743289. eCollection 2023. PPAR Res. 2023. PMID: 36875280 Free PMC article. Review.
-
Hyperhomocysteinemia lowers serum testosterone concentration via impairing testosterone production in Leydig cells.Cell Biol Toxicol. 2023 Dec;39(6):3077-3100. doi: 10.1007/s10565-023-09819-4. Epub 2023 Jul 27. Cell Biol Toxicol. 2023. PMID: 37495868
-
Pesticides and Male Fertility: A Dangerous Crosstalk.Metabolites. 2021 Nov 25;11(12):799. doi: 10.3390/metabo11120799. Metabolites. 2021. PMID: 34940557 Free PMC article. Review.
-
Exposure to 2,4-dichlorophenoxyacetic acid induced PPARβ-dependent disruption of glucose metabolism in HepG2 cells.Environ Sci Pollut Res Int. 2018 Jun;25(17):17050-17057. doi: 10.1007/s11356-018-1921-6. Epub 2018 Apr 9. Environ Sci Pollut Res Int. 2018. PMID: 29633193
-
Zoledronate dysregulates fatty acid metabolism in renal tubular epithelial cells to induce nephrotoxicity.Arch Toxicol. 2018 Jan;92(1):469-485. doi: 10.1007/s00204-017-2048-0. Epub 2017 Sep 4. Arch Toxicol. 2018. PMID: 28871336 Free PMC article.
References
-
- Abdellatif AG, Préat V, Vamecq J, Nilsson R, Roberfroid M (1990) Peroxisome proliferation and modulation of rat liver carcinogenesis by 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopin. Carcinogenesis 11:1899–1902 - PubMed
-
- Amer SM, Aly FA (2001) Genotoxic effect of 2,4-dichlorophenoxy acetic acid and its metabolite 2,4-dichlorophenol in mouse. Mutat Res 494:1–12 - PubMed
-
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV et al. (1989) Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem 264:10388–10395 - PubMed
-
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ (1998) Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem 273:5678–5684 - PubMed
-
- Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC (2001) Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 60:44–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

