Mechanical Forces Reshape Differentiation Cues That Guide Cardiomyogenesis
- PMID: 26838315
- PMCID: PMC4743530
- DOI: 10.1161/CIRCRESAHA.115.305139
Mechanical Forces Reshape Differentiation Cues That Guide Cardiomyogenesis
Abstract
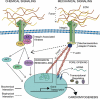
Soluble morphogen gradients have long been studied in the context of heart specification and patterning. However, recent data have begun to challenge the notion that long-standing in vivo observations are driven solely by these gradients alone. Evidence from multiple biological models, from stem cells to ex vivo biophysical assays, now supports a role for mechanical forces in not only modulating cell behavior but also inducing it de novo in a process termed mechanotransduction. Structural proteins that connect the cell to its niche, for example, integrins and cadherins, and that couple to other growth factor receptors, either directly or indirectly, seem to mediate these changes, although specific mechanistic details are still being elucidated. In this review, we summarize how the wingless (Wnt), transforming growth factor-β, and bone morphogenetic protein signaling pathways affect cardiomyogenesis and then highlight the interplay between each pathway and mechanical forces. In addition, we will outline the role of integrins and cadherins during cardiac development. For each, we will describe how the interplay could change multiple processes during cardiomyogenesis, including the specification of undifferentiated cells, the establishment of heart patterns to accomplish tube and chamber formation, or the maturation of myocytes in the fully formed heart.
Keywords: cadherins; heart; integrins; stem cells; transforming growth factor.
© 2016 American Heart Association, Inc.
Figures




Similar articles
-
Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma.Bone. 2008 Apr;42(4):669-80. doi: 10.1016/j.bone.2007.12.006. Epub 2007 Dec 27. Bone. 2008. PMID: 18294945
-
Role of smad- and wnt-dependent pathways in embryonic cardiac development.Stem Cells Dev. 2006 Feb;15(1):29-39. doi: 10.1089/scd.2006.15.29. Stem Cells Dev. 2006. PMID: 16522160
-
NFAT5 regulates the canonical Wnt pathway and is required for cardiomyogenic differentiation.Biochem Biophys Res Commun. 2012 Sep 28;426(3):317-23. doi: 10.1016/j.bbrc.2012.08.069. Epub 2012 Aug 23. Biochem Biophys Res Commun. 2012. PMID: 22935419
-
Integrin and cadherin signaling in bone: role and potential therapeutic targets.Trends Endocrinol Metab. 2014 Nov;25(11):567-75. doi: 10.1016/j.tem.2014.06.009. Epub 2014 Jul 14. Trends Endocrinol Metab. 2014. PMID: 25034128 Review.
-
Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance.Development. 2005 May;132(10):2251-62. doi: 10.1242/dev.01830. Development. 2005. PMID: 15857918 Review.
Cited by
-
Parametric optimization of culture chamber for cell mechanobiology research.Exp Biol Med (Maywood). 2023 Oct;248(20):1708-1717. doi: 10.1177/15353702231198079. Epub 2023 Oct 14. Exp Biol Med (Maywood). 2023. PMID: 37837381 Free PMC article.
-
Synthesis of aligned porous polyethylene glycol/silk fibroin/hydroxyapatite scaffolds for osteoinduction in bone tissue engineering.Stem Cell Res Ther. 2020 Dec 3;11(1):522. doi: 10.1186/s13287-020-02024-8. Stem Cell Res Ther. 2020. PMID: 33272329 Free PMC article.
-
Injectable pH Responsive Conductive Hydrogel for Intelligent Delivery of Metformin and Exosomes to Enhance Cardiac Repair after Myocardial Ischemia-Reperfusion Injury.Adv Sci (Weinh). 2025 Jun;12(24):e2410590. doi: 10.1002/advs.202410590. Epub 2025 Feb 18. Adv Sci (Weinh). 2025. PMID: 39965141 Free PMC article.
-
Current Understanding of the Pathways Involved in Adult Stem and Progenitor Cell Migration for Tissue Homeostasis and Repair.Stem Cell Rev Rep. 2016 Aug;12(4):421-37. doi: 10.1007/s12015-016-9663-7. Stem Cell Rev Rep. 2016. PMID: 27209167 Review.
-
Epithelial tension in the second heart field promotes mouse heart tube elongation.Nat Commun. 2017 Mar 30;8:14770. doi: 10.1038/ncomms14770. Nat Commun. 2017. PMID: 28357999 Free PMC article.
References
-
- Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. - PubMed
-
- Rogers KW, Schier AF. Morphogen gradients: from generation to interpretation. Annu Rev Cell Dev Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. - PubMed
-
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. - PubMed
-
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

