Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD
- PMID: 26838599
- PMCID: PMC4941147
- DOI: 10.1136/gutjnl-2015-310798
Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD
Abstract
Objective: Peroxisome proliferator-activated receptor α (PPARα) is a nuclear receptor expressed in tissues with high oxidative activity that plays a central role in metabolism. In this work, we investigated the effect of hepatocyte PPARα on non-alcoholic fatty liver disease (NAFLD).
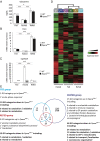
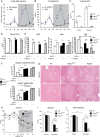
Design: We constructed a novel hepatocyte-specific PPARα knockout (Pparα(hep-/-)) mouse model. Using this novel model, we performed transcriptomic analysis following fenofibrate treatment. Next, we investigated which physiological challenges impact on PPARα. Moreover, we measured the contribution of hepatocytic PPARα activity to whole-body metabolism and fibroblast growth factor 21 production during fasting. Finally, we determined the influence of hepatocyte-specific PPARα deficiency in different models of steatosis and during ageing.
Results: Hepatocyte PPARα deletion impaired fatty acid catabolism, resulting in hepatic lipid accumulation during fasting and in two preclinical models of steatosis. Fasting mice showed acute PPARα-dependent hepatocyte activity during early night, with correspondingly increased circulating free fatty acids, which could be further stimulated by adipocyte lipolysis. Fasting led to mild hypoglycaemia and hypothermia in Pparα(hep-/-) mice when compared with Pparα(-/-) mice implying a role of PPARα activity in non-hepatic tissues. In agreement with this observation, Pparα(-/-) mice became overweight during ageing while Pparα(hep-/-) remained lean. However, like Pparα(-/-) mice, Pparα(hep-/-) fed a standard diet developed hepatic steatosis in ageing.
Conclusions: Altogether, these findings underscore the potential of hepatocyte PPARα as a drug target for NAFLD.
Keywords: GENE EXPRESSION; LIPID METABOLISM; NONALCOHOLIC STEATOHEPATITIS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures







Comment in
-
Hepatic-specific PPARα-FGF21 action in NAFLD.Gut. 2016 Jul;65(7):1075-6. doi: 10.1136/gutjnl-2016-311408. Epub 2016 Mar 18. Gut. 2016. PMID: 26992428 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
