Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response
- PMID: 26838600
- PMCID: PMC5531227
- DOI: 10.1136/gutjnl-2015-309897
Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response
Abstract
Objective: To identify a causal mechanism responsible for the enhancement of insulin resistance and hyperglycaemia following periodontitis in mice fed a fat-enriched diet.
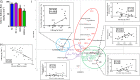
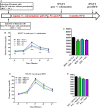
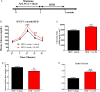
Design: We set-up a unique animal model of periodontitis in C57Bl/6 female mice by infecting the periodontal tissue with specific and alive pathogens like Porphyromonas gingivalis (Pg), Fusobacterium nucleatum and Prevotella intermedia. The mice were then fed with a diabetogenic/non-obesogenic fat-enriched diet for up to 3 months. Alveolar bone loss, periodontal microbiota dysbiosis and features of glucose metabolism were quantified. Eventually, adoptive transfer of cervical (regional) and systemic immune cells was performed to demonstrate the causal role of the cervical immune system.
Results: Periodontitis induced a periodontal microbiota dysbiosis without mainly affecting gut microbiota. The disease concomitantly impacted on the regional and systemic immune response impairing glucose metabolism. The transfer of cervical lymph-node cells from infected mice to naive recipients guarded against periodontitis-aggravated metabolic disease. A treatment with inactivated Pg prior to the periodontal infection induced specific antibodies against Pg and protected the mouse from periodontitis-induced dysmetabolism. Finally, a 1-month subcutaneous chronic infusion of low rates of lipopolysaccharides from Pg mimicked the impact of periodontitis on immune and metabolic parameters.
Conclusions: We identified that insulin resistance in the high-fat fed mouse is enhanced by pathogen-induced periodontitis. This is caused by an adaptive immune response specifically directed against pathogens and associated with a periodontal dysbiosis.
Keywords: BACTERIAL INFECTION; BACTERIAL PATHOGENESIS; DIABETES MELLITUS; DIET; IMMUNE RESPONSE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
