Integrin signalling regulates the expansion of neuroepithelial progenitors and neurogenesis via Wnt7a and Decorin
- PMID: 26838601
- PMCID: PMC4742793
- DOI: 10.1038/ncomms10354
Integrin signalling regulates the expansion of neuroepithelial progenitors and neurogenesis via Wnt7a and Decorin
Abstract
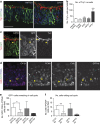
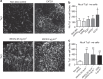
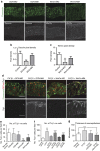
Development of the cerebral cortex requires regulation of proliferation and differentiation of neural stem cells and a diverse range of progenitors. Recent work suggests a role for extracellular matrix (ECM) and the major family of ECM receptors, the integrins. Here we show that enhancing integrin beta-1 signalling, by expressing a constitutively active integrin beta-1 (CA*β1) in the embryonic chick mesencephalon, enhances neurogenesis and increases the number of mitotic cells dividing away from the ventricular surface, analogous to sub-apical progenitors in mouse. Only non-integrin-expressing neighbouring cells (lacking CA*β1) contributed to the increased neurogenesis. Transcriptome analysis reveals upregulation of Wnt7a within the CA*β1 cells and upregulation of the ECM protein Decorin in the neighbouring non-expressing cells. Experiments using inhibitors in explant models and genetic knock-downs in vivo reveal an integrin-Wnt7a-Decorin pathway that promotes proliferation and differentiation of neuroepithelial cells, and identify Decorin as a novel neurogenic factor in the central nervous system.
Figures






Similar articles
-
Effect of transforming growth factor-beta on decorin and beta1 integrin expression during muscle development in chickens.Poult Sci. 2006 Feb;85(2):326-32. doi: 10.1093/ps/85.2.326. Poult Sci. 2006. PMID: 16523634
-
β1 integrin is a crucial regulator of pancreatic β-cell expansion.Development. 2013 Aug;140(16):3360-72. doi: 10.1242/dev.098533. Epub 2013 Jul 17. Development. 2013. PMID: 23863477 Free PMC article.
-
Stage-specific functions of Semaphorin7A during adult hippocampal neurogenesis rely on distinct receptors.Nat Commun. 2017 Mar 10;8:14666. doi: 10.1038/ncomms14666. Nat Commun. 2017. PMID: 28281529 Free PMC article.
-
Small leucine rich proteoglycan family regulates multiple signalling pathways in neural development and maintenance.Dev Growth Differ. 2012 Apr;54(3):327-40. doi: 10.1111/j.1440-169X.2012.01339.x. Dev Growth Differ. 2012. PMID: 22524604 Review.
-
From neuroepithelial cells to neurons: changes in the physiological properties of neuroepithelial stem cells.Arch Biochem Biophys. 2013 Jun;534(1-2):64-70. doi: 10.1016/j.abb.2012.07.016. Epub 2012 Aug 7. Arch Biochem Biophys. 2013. PMID: 22892549 Review.
Cited by
-
Hindbrain boundaries as niches of neural progenitor and stem cells regulated by the extracellular matrix proteoglycan chondroitin sulphate.Development. 2024 Feb 15;151(4):dev201934. doi: 10.1242/dev.201934. Epub 2024 Feb 13. Development. 2024. PMID: 38251863 Free PMC article.
-
Human dermal fibroblast-derived secretory proteins for regulating nerve restoration: A bioinformatic approach.Skin Res Technol. 2024 Jun;30(6):e13810. doi: 10.1111/srt.13810. Skin Res Technol. 2024. PMID: 38887125 Free PMC article.
-
Opposite Roles of Wnt7a and Sfrp1 in Modulating Proper Development of Neural Progenitors in the Mouse Cerebral Cortex.Front Mol Neurosci. 2018 Jul 17;11:247. doi: 10.3389/fnmol.2018.00247. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30065628 Free PMC article.
-
Extracellular Matrix Proteins Differentiate Postnatal Mouse Retina Neurospheres into Neurons or Glia Profiles.Neurochem Res. 2025 Jul 21;50(4):242. doi: 10.1007/s11064-025-04500-0. Neurochem Res. 2025. PMID: 40691371
-
Transcriptional Regulators and Human-Specific/Primate-Specific Genes in Neocortical Neurogenesis.Int J Mol Sci. 2020 Jun 29;21(13):4614. doi: 10.3390/ijms21134614. Int J Mol Sci. 2020. PMID: 32610533 Free PMC article. Review.
References
-
- Hirabayashi Y. et al.. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131, 2791–2801 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

