A Novel Secreted Protein, MYR1, Is Central to Toxoplasma's Manipulation of Host Cells
- PMID: 26838724
- PMCID: PMC4742717
- DOI: 10.1128/mBio.02231-15
A Novel Secreted Protein, MYR1, Is Central to Toxoplasma's Manipulation of Host Cells
Erratum in
-
Erratum for Franco et al., "A Novel Secreted Protein, MYR1, Is Central to Toxoplasma's Manipulation of Host Cells".mBio. 2018 Oct 2;9(5):e01869-18. doi: 10.1128/mBio.01869-18. mBio. 2018. PMID: 30279290 Free PMC article. No abstract available.
Abstract
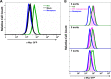
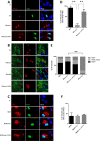
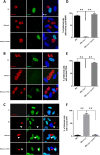
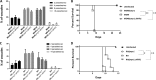
The intracellular protozoan Toxoplasma gondii dramatically reprograms the transcriptome of host cells it infects, including substantially up-regulating the host oncogene c-myc. By applying a flow cytometry-based selection to infected mouse cells expressing green fluorescent protein fused to c-Myc (c-Myc-GFP), we isolated mutant tachyzoites defective in this host c-Myc up-regulation. Whole-genome sequencing of three such mutants led to the identification of MYR1 (Myc regulation 1; TGGT1_254470) as essential for c-Myc induction. MYR1 is a secreted protein that requires TgASP5 to be cleaved into two stable portions, both of which are ultimately found within the parasitophorous vacuole and at the parasitophorous vacuole membrane. Deletion of MYR1 revealed that in addition to its requirement for c-Myc up-regulation, the MYR1 protein is needed for the ability of Toxoplasma tachyzoites to modulate several other important host pathways, including those mediated by the dense granule effectors GRA16 and GRA24. This result, combined with its location at the parasitophorous vacuole membrane, suggested that MYR1 might be a component of the machinery that translocates Toxoplasma effectors from the parasitophorous vacuole into the host cytosol. Support for this possibility was obtained by showing that transit of GRA24 to the host nucleus is indeed MYR1-dependent. As predicted by this pleiotropic phenotype, parasites deficient in MYR1 were found to be severely attenuated in a mouse model of infection. We conclude, therefore, that MYR1 is a novel protein that plays a critical role in how Toxoplasma delivers effector proteins to the infected host cell and that this is crucial to virulence.
Importance: Toxoplasma gondii is an important human pathogen and a model for the study of intracellular parasitism. Infection of the host cell with Toxoplasma tachyzoites involves the introduction of protein effectors, including many that are initially secreted into the parasitophorous vacuole but must ultimately translocate to the host cell cytosol to function. The work reported here identified a novel protein that is required for this translocation. These results give new insight into a very unusual cell biology process as well as providing a potential handle on a pathway that is necessary for virulence and, therefore, a new potential target for chemotherapy.
Copyright © 2016 Franco et al.
Figures





Similar articles
-
Coimmunoprecipitation with MYR1 Identifies Three Additional Proteins within the Toxoplasma gondii Parasitophorous Vacuole Required for Translocation of Dense Granule Effectors into Host Cells.mSphere. 2020 Feb 19;5(1):e00858-19. doi: 10.1128/mSphere.00858-19. mSphere. 2020. PMID: 32075880 Free PMC article.
-
Identification of a novel protein complex essential for effector translocation across the parasitophorous vacuole membrane of Toxoplasma gondii.PLoS Pathog. 2018 Jan 22;14(1):e1006828. doi: 10.1371/journal.ppat.1006828. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29357375 Free PMC article.
-
Toxoplasma Uses GRA16 To Upregulate Host c-Myc.mSphere. 2020 Jun 24;5(3):e00402-20. doi: 10.1128/mSphere.00402-20. mSphere. 2020. PMID: 32581080 Free PMC article.
-
Toxoplasma's ways of manipulating the host transcriptome via secreted effectors.Curr Opin Microbiol. 2015 Aug;26:24-31. doi: 10.1016/j.mib.2015.04.003. Epub 2015 Apr 24. Curr Opin Microbiol. 2015. PMID: 25912924 Review.
-
Seizing control: How dense granule effector proteins enable Toxoplasma to take charge.Mol Microbiol. 2021 Mar;115(3):466-477. doi: 10.1111/mmi.14679. Epub 2021 Feb 6. Mol Microbiol. 2021. PMID: 33400323 Free PMC article. Review.
Cited by
-
Theileria's Strategies and Effector Mechanisms for Host Cell Transformation: From Invasion to Immortalization.Front Cell Dev Biol. 2021 Apr 20;9:662805. doi: 10.3389/fcell.2021.662805. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33959614 Free PMC article. Review.
-
Secreted protein kinases regulate cyst burden during chronic toxoplasmosis.Cell Microbiol. 2017 Feb;19(2):10.1111/cmi.12651. doi: 10.1111/cmi.12651. Epub 2016 Aug 25. Cell Microbiol. 2017. PMID: 27450947 Free PMC article.
-
Genome-wide screens identify Toxoplasma gondii determinants of parasite fitness in IFNγ-activated murine macrophages.Nat Commun. 2020 Oct 16;11(1):5258. doi: 10.1038/s41467-020-18991-8. Nat Commun. 2020. PMID: 33067458 Free PMC article.
-
Inhibition of Nitric Oxide Production in Activated Macrophages Caused by Toxoplasma gondii Infection Occurs by Distinct Mechanisms in Different Mouse Macrophage Cell Lines.Front Microbiol. 2018 Aug 20;9:1936. doi: 10.3389/fmicb.2018.01936. eCollection 2018. Front Microbiol. 2018. PMID: 30177926 Free PMC article.
-
Efficient Gene Knockout and Knockdown Systems in Neospora caninum Enable Rapid Discovery and Functional Assessment of Novel Proteins.mSphere. 2022 Feb 23;7(1):e0089621. doi: 10.1128/msphere.00896-21. Epub 2022 Jan 12. mSphere. 2022. PMID: 35019667 Free PMC article.
References
-
- Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, Soldati-Favre D, Takeda K. 2009. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med 206:2747–2760. doi:10.1084/jem.20091703. - DOI - PMC - PubMed
-
- Jensen KD, Hu K, Whitmarsh RJ, Hassan MA, Julien L, Lu D, Chen L, Hunter CA, Saeij JP. 2013. Toxoplasma gondii rhoptry 16 kinase promotes host resistance to oral infection and intestinal inflammation only in the context of the dense granule protein GRA15. Infect Immun 81:2156–2167. doi:10.1128/IAI.01185-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI73756/AI/NIAID NIH HHS/United States
- P30 NS069375/NS/NINDS NIH HHS/United States
- P01 HG000205/HG/NHGRI NIH HHS/United States
- F31 AI120649/AI/NIAID NIH HHS/United States
- K08 AI102946/AI/NIAID NIH HHS/United States
- T32 AI007328/AI/NIAID NIH HHS/United States
- R01 AI073756/AI/NIAID NIH HHS/United States
- S10 RR025518/RR/NCRR NIH HHS/United States
- R01 AI112962/AI/NIAID NIH HHS/United States
- NS069375/NS/NINDS NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- R21 AI112962/AI/NIAID NIH HHS/United States
- S10RR025518-01/RR/NCRR NIH HHS/United States
- K08AI102946-01/AI/NIAID NIH HHS/United States
- P01-35HG000205/HG/NHGRI NIH HHS/United States
- R01 AI074953/AI/NIAID NIH HHS/United States
- F32 HG000205/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

