Evaluation of role of Notch3 signaling pathway in human lung cancer cells
- PMID: 26838758
- PMCID: PMC11818984
- DOI: 10.1007/s00432-016-2117-4
Evaluation of role of Notch3 signaling pathway in human lung cancer cells
Abstract
There is still a debate on the extent to which Notch3 signaling is involved in lung carcinogenesis and whether such function is dependent on cancer type or not.
Purpose: To evaluate Notch3 expression in different types of human lung cancer cells.
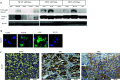
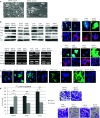
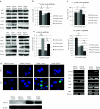
Methods: Notch3 was detected in human lung cancer cell lines and in tissues. Then, small interfering RNA (siRNA) was used to down-regulate the expression of Notch3 in H69AR small cell lung carcinoma (SCLC) cells; two non-small cell lung carcinoma (NSCLC) cells; A549 adenocarcinoma (ADC); and H2170 squamous cell carcinoma (SCC). In addition, Notch3 intracellular domain (N3ICD) plasmid was transfected into H1688 human SCLC cells. We observed the effect of deregulating Notch3 signaling on the following cell properties: Notch-related proteins, cell morphology, adhesion, epithelial-mesenchymal transition (EMT), motility, proliferation and neuroendocrine (NE) features of SCLC.
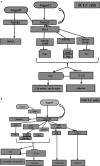
Results: Notch3 is mainly expressed in NSCLC, and the expression of Notch1, Hes1 and Jagged1 is affected by Notch3. Notch3 has opposite functions in SCLC and NSCLC, being a tumor suppressor in the former and tumor promoting in the latter, in the context of cell adhesion, EMT and motility. Regarding cell proliferation, we found that inhibiting Notch3 in NSCLC decreases cell proliferation and induces apoptosis in NSCLC. Notch3 has no effect on cell proliferation or NE features of SCLC.
Conclusion: Notch3 signaling in lung carcinoma is dependent on cell type. In SCLC, Notch3 behaves as a tumor suppressor pathway, while in NSCLC it acts as a tumor-promoting pathway.
Keywords: Human lung cancer; Notch3 intracellular domain (N3ICD) plasmid; Notch3 signaling; Small interfering RNA (siRNA).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Bunn PA Jr (2012) Worldwide overview of the current status of lung cancer diagnosis and treatment. Arch Pathol Lab Med 136:1478–1481 - PubMed
-
- Collins BJ, Kleeberger W, Ball DW (2004) Notch in lung development and lung cancer. Semin Cancer Biol 14(5):357–364 - PubMed
-
- Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, Roberts JR, Carbone DP (2000) Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst 92:1355–1357 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

