Mutual Regulation of Epicardial Adipose Tissue and Myocardial Redox State by PPAR-γ/Adiponectin Signalling
- PMID: 26838789
- PMCID: PMC4772814
- DOI: 10.1161/CIRCRESAHA.115.307856
Mutual Regulation of Epicardial Adipose Tissue and Myocardial Redox State by PPAR-γ/Adiponectin Signalling
Abstract
Rationale: Adiponectin has anti-inflammatory effects in experimental models, but its role in the regulation of myocardial redox state in humans is unknown. Although adiponectin is released from epicardial adipose tissue (EpAT), it is unclear whether it exerts any paracrine effects on the human myocardium.
Objective: To explore the cross talk between EpAT-derived adiponectin and myocardial redox state in the human heart.
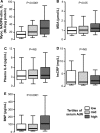
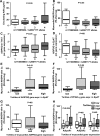
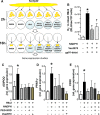
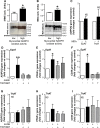
Methods and results: EpAT and atrial myocardium were obtained from 306 patients undergoing coronary artery bypass grafting. Functional genetic polymorphisms that increase ADIPOQ expression (encoding adiponectin) led to reduced myocardial nicotinamide adenine dinucleotide phosphate oxidase-derived O2 (-), whereas circulating adiponectin and ADIPOQ expression in EpAT were associated with elevated myocardial O2 (-). In human atrial tissue, we demonstrated that adiponectin suppresses myocardial nicotinamide adenine dinucleotide phosphate oxidase activity, by preventing AMP kinase-mediated translocation of Rac1 and p47(phox) from the cytosol to the membranes. Induction of O2 (-) production in H9C2 cardiac myocytes led to the release of a transferable factor able to induce peroxisome proliferator-activated receptor-γ-mediated upregulation of ADIPOQ expression in cocultured EpAT. Using a NOX2 transgenic mouse and a pig model of rapid atrial pacing, we found that oxidation products (such as 4-hydroxynonenal) released from the heart trigger peroxisome proliferator-activated receptor-γ-mediated upregulation of ADIPOQ in EpAT.
Conclusions: We demonstrate for the first time in humans that adiponectin directly decreases myocardial nicotinamide adenine dinucleotide phosphate oxidase activity via endocrine or paracrine effects. Adiponectin expression in EpAT is controlled by paracrine effects of oxidation products released from the heart. These effects constitute a novel defense mechanism of the heart against myocardial oxidative stress.
Keywords: adiponectin; adipose tissue; myocardium; obesity; oxidative stress.
© 2016 The Authors.
Figures



References
-
- Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. - PubMed
-
- Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. - PubMed
-
- Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U, Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. - PubMed
-
- Antoniades C, Demosthenous M, Reilly S, et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59:60–70. doi: 10.1016/j.jacc.2011.08.062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

