Interventions to increase HPV vaccination coverage: A systematic review
- PMID: 26838959
- PMCID: PMC4964671
- DOI: 10.1080/21645515.2015.1125055
Interventions to increase HPV vaccination coverage: A systematic review
Abstract
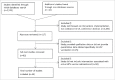
We reviewed intervention studies designed to increase human papillomavirus (HPV) vaccination coverage to further understand the impact interventions can have on HPV vaccination coverage. We searched 5 databases for intervention studies published from June 2006 to May 2015. Studies were included if they quantitatively measured HPV vaccination coverage as an outcome and were conducted in the United States. We abstracted outcomes, methods, and results from each study and classified by type of intervention conducted. Findings from 34 studies suggest many types of intervention strategies can increase HPV vaccination coverage in different settings, and with modest cost. Interventions were effective especially when implemented in combination at both provider and community levels. However, not all interventions showed significant effects on coverage. More research is needed to identify the best methods for widespread implementation of effective strategies.
Keywords: HPV; coverage; human papillomavirus; interventions; vaccination.
Figures

References
-
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, Su J, Xu F, Winstock H. Sexually transmitted infections among US women and men: prevalence and incidence estimates 2008. Sex Transm Dis 2013; 40(3):187-93; PMID:23403598 - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Chesson H, Curtis CR, Gee J, Bocchini JA, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report (MMWR) 2014; 63(RR05):1-30 - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2007; 56(RR02):1-24 - PubMed
-
- Centers for Disease Control and Prevention FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2010; 59(20):626-9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
