Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders
- PMID: 26839058
- PMCID: PMC4742909
- DOI: 10.1038/ncomms10594
Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders
Erratum in
-
Corrigendum: Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders.Nat Commun. 2016 Apr 20;7:11466. doi: 10.1038/ncomms11466. Nat Commun. 2016. PMID: 27095349 Free PMC article. No abstract available.
Abstract
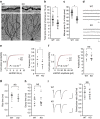
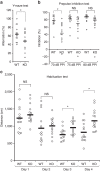
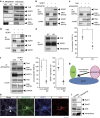
Intracellular trafficking of receptor proteins is essential for neurons to detect various extracellular factors during the formation and refinement of neural circuits. However, the precise mechanisms underlying the trafficking of neurotrophin receptors to synapses remain elusive. Here, we demonstrate that a brain-enriched sorting nexin, ARHGAP33, is a new type of regulator for the intracellular trafficking of TrkB, a high-affinity receptor for brain-derived neurotrophic factor. ARHGAP33 knockout (KO) mice exhibit reduced expression of synaptic TrkB, impaired spine development and neuropsychiatric disorder-related behavioural abnormalities. These deficits are rescued by specific pharmacological enhancement of TrkB signalling in ARHGAP33 KO mice. Mechanistically, ARHGAP33 interacts with SORT1 to cooperatively regulate TrkB trafficking. Human ARHGAP33 is associated with brain phenotypes and reduced SORT1 expression is found in patients with schizophrenia. We propose that ARHGAP33/SORT1-mediated TrkB trafficking is essential for synapse development and that the dysfunction of this mechanism may be a new molecular pathology of neuropsychiatric disorders.
Figures





Similar articles
-
SNX26, a GTPase-activating protein for Cdc42, interacts with PSD-95 protein and is involved in activity-dependent dendritic spine formation in mature neurons.J Biol Chem. 2013 Oct 11;288(41):29453-66. doi: 10.1074/jbc.M113.468801. Epub 2013 Sep 3. J Biol Chem. 2013. PMID: 24003235 Free PMC article.
-
NOMA-GAP/ARHGAP33 regulates synapse development and autistic-like behavior in the mouse.Mol Psychiatry. 2015 Sep;20(9):1120-31. doi: 10.1038/mp.2015.42. Epub 2015 Apr 14. Mol Psychiatry. 2015. PMID: 25869807
-
Reduced mastication during growth inhibits cognitive function by affecting trigeminal ganglia and modulating Wnt signaling pathway and ARHGAP33 molecular transmission.Neuropeptides. 2023 Dec;102:102370. doi: 10.1016/j.npep.2023.102370. Epub 2023 Aug 17. Neuropeptides. 2023. PMID: 37634443
-
Neuronal and brain morphological changes in animal models of schizophrenia.Behav Brain Res. 2016 Mar 15;301:190-203. doi: 10.1016/j.bbr.2015.12.034. Epub 2015 Dec 29. Behav Brain Res. 2016. PMID: 26738967 Review.
-
Sorting nexin 11 knockout mice exhibit enhanced thermosensing behaviour.Genes Brain Behav. 2020 Jul;19(6):e12625. doi: 10.1111/gbb.12625. Epub 2019 Dec 15. Genes Brain Behav. 2020. PMID: 31730264 Review.
Cited by
-
Pathogenic POGZ mutation causes impaired cortical development and reversible autism-like phenotypes.Nat Commun. 2020 Feb 26;11(1):859. doi: 10.1038/s41467-020-14697-z. Nat Commun. 2020. PMID: 32103003 Free PMC article.
-
Zebrafish myo7aa affects congenital hearing by regulating Rho-GTPase signaling.Front Mol Neurosci. 2024 Jul 15;17:1405109. doi: 10.3389/fnmol.2024.1405109. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39081296 Free PMC article.
-
Trends in big data analyses by multicenter collaborative translational research in psychiatry.Psychiatry Clin Neurosci. 2022 Jan;76(1):1-14. doi: 10.1111/pcn.13311. Psychiatry Clin Neurosci. 2022. PMID: 34716732 Free PMC article. Review.
-
A CRISPR screen in intestinal epithelial cells identifies novel factors for polarity and apical transport.Elife. 2023 Jan 20;12:e80135. doi: 10.7554/eLife.80135. Elife. 2023. PMID: 36661306 Free PMC article.
-
Identification of a Robust Five-Gene Risk Model in Prostate Cancer: A Robust Likelihood-Based Survival Analysis.Int J Genomics. 2020 May 27;2020:1097602. doi: 10.1155/2020/1097602. eCollection 2020. Int J Genomics. 2020. PMID: 32566639 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

