Stimulated Raman scattering microscopy: an emerging tool for drug discovery
- PMID: 26839248
- PMCID: PMC4839273
- DOI: 10.1039/c5cs00693g
Stimulated Raman scattering microscopy: an emerging tool for drug discovery
Abstract
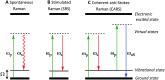
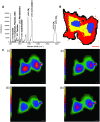
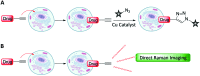
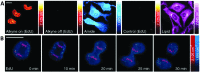
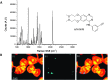
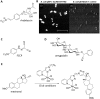
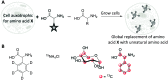
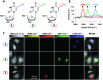
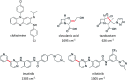
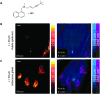
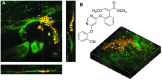
Optical microscopy techniques have emerged as a cornerstone of biomedical research, capable of probing the cellular functions of a vast range of substrates, whilst being minimally invasive to the cells or tissues of interest. Incorporating biological imaging into the early stages of the drug discovery process can provide invaluable information about drug activity within complex disease models. Spontaneous Raman spectroscopy has been widely used as a platform for the study of cells and their components based on chemical composition; but slow acquisition rates, poor resolution and a lack of sensitivity have hampered further development. A new generation of stimulated Raman techniques is emerging which allows the imaging of cells, tissues and organisms at faster acquisition speeds, and with greater resolution and sensitivity than previously possible. This review focuses on the development of stimulated Raman scattering (SRS), and covers the use of bioorthogonal tags to enhance sample detection, and recent applications of both spontaneous Raman and SRS as novel imaging platforms to facilitate the drug discovery process.
Figures












References
-
- Kola I., Landis J. Nat. Rev. Drug Discovery. 2004;3:711–716. - PubMed
-
- Paul S. M., Mytelka D. S., Dunwiddie C. T., Persinger C. C., Munos B. H., Lindborg S. R., Schacht A. L. Nat. Rev. Drug Discovery. 2010;9:203–214. - PubMed
-
- Ocana A., Pandiella A., Siu L. L., Tannock I. F. Nat. Rev. Clin. Oncol. 2011;8:200–209. - PubMed
-
- Swinney D. C., Anthony J. Nat. Rev. Drug Discovery. 2011;10:507–519. - PubMed
-
- Moreno L., Pearson A. D. Expert Opin. Drug Discovery. 2013;8:363–368. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

