Carbon Nanotube and Asbestos Exposures Induce Overlapping but Distinct Profiles of Lung Pathology in Non-Swiss Albino CF-1 Mice
- PMID: 26839332
- PMCID: PMC4976500
- DOI: 10.1177/0192623315620587
Carbon Nanotube and Asbestos Exposures Induce Overlapping but Distinct Profiles of Lung Pathology in Non-Swiss Albino CF-1 Mice
Abstract
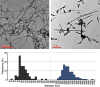
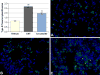
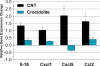
Carbon nanotubes (CNTs) are emerging as important occupational and environmental toxicants owing to their increasing prevalence and potential to be inhaled as airborne particles. CNTs are a concern because of their similarities to asbestos, which include fibrous morphology, high aspect ratio, and biopersistence. Limitations in research models have made it difficult to experimentally ascertain the risk of CNT exposures to humans and whether these may lead to lung diseases classically associated with asbestos, such as mesothelioma and fibrosis. In this study, we sought to comprehensively compare profiles of lung pathology in mice following repeated exposures to multiwall CNTs or crocidolite asbestos (CA). We show that both exposures resulted in granulomatous inflammation and increased interstitial collagen; CA exposures caused predominantly bronchoalveolar hyperplasia, whereas CNT exposures caused alveolar hyperplasia of type II pneumocytes (T2Ps). T2Ps isolated from CNT-exposed lungs were found to have upregulated proinflammatory genes, including interleukin 1ß (IL-1ß), in contrast to those from CA exposed. Immunostaining in tissue showed that while both toxicants increased IL-1ß protein expression in lung cells, T2P-specific IL-1ß increases were greater following CNT exposure. These results suggest related but distinct mechanisms of action by CNTs versus asbestos which may lead to different outcomes in the 2 exposure types.
Keywords: carbon nanotubes; comparative pathology; crocidolite asbestos; fiber toxicology; lung; mouse.
© The Author(s) 2016.
Figures







Similar articles
-
Comparative proteomics and pulmonary toxicity of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice.Toxicol Sci. 2011 Mar;120(1):123-35. doi: 10.1093/toxsci/kfq363. Epub 2010 Dec 6. Toxicol Sci. 2011. PMID: 21135415 Free PMC article.
-
Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos.Part Fibre Toxicol. 2012 Apr 10;9:10. doi: 10.1186/1743-8977-9-10. Part Fibre Toxicol. 2012. PMID: 22490147 Free PMC article.
-
Multiwalled carbon nanotube-induced pulmonary inflammatory and fibrotic responses and genomic changes following aspiration exposure in mice: A 1-year postexposure study.J Toxicol Environ Health A. 2016;79(8):352-66. doi: 10.1080/15287394.2016.1159635. Epub 2016 Apr 19. J Toxicol Environ Health A. 2016. PMID: 27092743 Free PMC article.
-
Pulmonary toxicity of carbon nanotubes and asbestos - similarities and differences.Adv Drug Deliv Rev. 2013 Dec;65(15):2078-86. doi: 10.1016/j.addr.2013.07.014. Epub 2013 Jul 27. Adv Drug Deliv Rev. 2013. PMID: 23899865 Review.
-
A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks.Crit Rev Toxicol. 2006 Mar;36(3):189-217. doi: 10.1080/10408440600570233. Crit Rev Toxicol. 2006. PMID: 16686422 Review.
Cited by
-
Cytotoxicity and Characterization of Ultrafine Particles from Desktop Three-Dimensional Printers with Multiple Filaments.Toxics. 2023 Aug 22;11(9):720. doi: 10.3390/toxics11090720. Toxics. 2023. PMID: 37755731 Free PMC article.
-
Carbon Nanotube Immunotoxicity in Alveolar Epithelial Type II Cells Is Mediated by Physical Contact-Independent Cell-Cell Interaction with Macrophages as Demonstrated in an Optimized Air-Liquid Interface (ALI) Coculture Model.Nanomaterials (Basel). 2024 Jul 29;14(15):1273. doi: 10.3390/nano14151273. Nanomaterials (Basel). 2024. PMID: 39120378 Free PMC article.
-
Differential modulation of lung aquaporins among other pathophysiological markers in acute (Cl2 gas) and chronic (carbon nanoparticles, cigarette smoke) respiratory toxicity mouse models.Front Physiol. 2022 Sep 28;13:880815. doi: 10.3389/fphys.2022.880815. eCollection 2022. Front Physiol. 2022. PMID: 36246134 Free PMC article.
-
Critical values for dimensional parameters of mesotheliomagenic mineral fibers: evidence from the dimensions and rigidity of MWCNT.Front Toxicol. 2025 Apr 22;7:1568513. doi: 10.3389/ftox.2025.1568513. eCollection 2025. Front Toxicol. 2025. PMID: 40330553 Free PMC article.
-
Oro-Respiratory Dysbiosis and Its Modulatory Effect on Lung Mucosal Toxicity during Exposure or Co-Exposure to Carbon Nanotubes and Cigarette Smoke.Nanomaterials (Basel). 2024 Feb 4;14(3):314. doi: 10.3390/nano14030314. Nanomaterials (Basel). 2024. PMID: 38334585 Free PMC article.
References
-
- Adamson IY, Bowden DH. Response of mouse lung to crocidolite asbestos. 2. Pulmonary fibrosis after long fibres. The Journal of pathology. 1987;152(2):109–117. - PubMed
-
- Akiyama I, Ogami A, Oyabu T, Yamato H, Morimoto Y, Tanaka I. Pulmonary effects and biopersistence of deposited silicon carbide whisker after 1-year inhalation in rats. Inhalation toxicology. 2007;19(2):141–147. - PubMed
-
- Birch ME. Monitoring of diesel particulate exhaust in the workplace. In: Schlecht PC, O’Connor PF, editors. NIOSH Manual of Analytical Methods (NMAM) 4th. Department of Health and Human Services, Public Health Service, Center for Disease Control and Prevention, National Institute for Occupational Safety and Health. DHHS(NIOSH); 154, Cincinnati, Ohio: 2004a. Third Supplement to NMAM.
-
- Birch ME. NIOSH Method 5040 update. In: Schlecht PC, O’Connor PF, editors. NIOSH Manual of Analytical Methods (NMAM) 4th. Department of Health and Human Services, Public Health Service, Center for Disease Control and Prevention, National Institute for Occupational Safety and Health. DHHS(NIOSH); 154, Cincinnati, Ohio: 2004b. Third Supplement to NMAM.
-
- Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdörster G, Harkema JR, Bramble LA, Kavanagh TJ, Botta D, Nei A, Pinkerton KE. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environmental health perspectives. 2013;121(6):676–682. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

