Differential vesicular sorting of AMPA and GABAA receptors
- PMID: 26839408
- PMCID: PMC4763724
- DOI: 10.1073/pnas.1525726113
Differential vesicular sorting of AMPA and GABAA receptors
Abstract
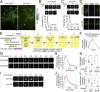
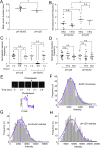
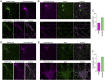
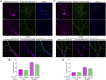
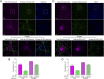
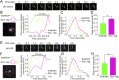
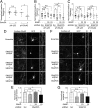
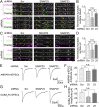
In mature neurons AMPA receptors cluster at excitatory synapses primarily on dendritic spines, whereas GABAA receptors cluster at inhibitory synapses mainly on the soma and dendritic shafts. The molecular mechanisms underlying the precise sorting of these receptors remain unclear. By directly studying the constitutive exocytic vesicles of AMPA and GABAA receptors in vitro and in vivo, we demonstrate that they are initially sorted into different vesicles in the Golgi apparatus and inserted into distinct domains of the plasma membrane. These insertions are dependent on distinct Rab GTPases and SNARE complexes. The insertion of AMPA receptors requires SNAP25-syntaxin1A/B-VAMP2 complexes, whereas insertion of GABAA receptors relies on SNAP23-syntaxin1A/B-VAMP2 complexes. These SNARE complexes affect surface targeting of AMPA or GABAA receptors and synaptic transmission. Our studies reveal vesicular sorting mechanisms controlling the constitutive exocytosis of AMPA and GABAA receptors, which are critical for the regulation of excitatory and inhibitory responses in neurons.
Keywords: AMPA receptor; GABAA receptor; SNARE; TIRFM; constitutive exocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

