Implications of recurrent disturbance for genetic diversity
- PMID: 26839689
- PMCID: PMC4725449
- DOI: 10.1002/ece3.1948
Implications of recurrent disturbance for genetic diversity
Abstract
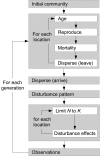
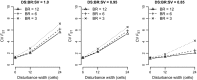
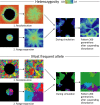
Exploring interactions between ecological disturbance, species' abundances and community composition provides critical insights for ecological dynamics. While disturbance is also potentially an important driver of landscape genetic patterns, the mechanisms by which these patterns may arise by selective and neutral processes are not well-understood. We used simulation to evaluate the relative importance of disturbance regime components, and their interaction with demographic and dispersal processes, on the distribution of genetic diversity across landscapes. We investigated genetic impacts of variation in key components of disturbance regimes and spatial patterns that are likely to respond to climate change and land management, including disturbance size, frequency, and severity. The influence of disturbance was mediated by dispersal distance and, to a limited extent, by birth rate. Nevertheless, all three disturbance regime components strongly influenced spatial and temporal patterns of genetic diversity within subpopulations, and were associated with changes in genetic structure. Furthermore, disturbance-induced changes in temporal population dynamics and the spatial distribution of populations across the landscape resulted in disrupted isolation by distance patterns among populations. Our results show that forecast changes in disturbance regimes have the potential to cause major changes to the distribution of genetic diversity within and among populations. We highlight likely scenarios under which future changes to disturbance size, severity, or frequency will have the strongest impacts on population genetic patterns. In addition, our results have implications for the inference of biological processes from genetic data, because the effects of dispersal on genetic patterns were strongly mediated by disturbance regimes.
Keywords: Allele surfing; disturbance regimes; range expansion; recolonization; simulation.
Figures



Similar articles
-
When can refuges mediate the genetic effects of fire regimes? A simulation study of the effects of topography and weather on neutral and adaptive genetic diversity in fire-prone landscapes.Mol Ecol. 2017 Oct;26(19):4935-4954. doi: 10.1111/mec.14250. Epub 2017 Aug 16. Mol Ecol. 2017. PMID: 28734110
-
Disturbance size and frequency mediate the coexistence of benthic spatial competitors.Ecology. 2020 Jan;101(1):e02904. doi: 10.1002/ecy.2904. Epub 2019 Nov 6. Ecology. 2020. PMID: 31562771
-
Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia's arid zone, the golden perch (Macquaria ambigua).Mol Ecol. 2010 Nov;19(21):4723-37. doi: 10.1111/j.1365-294X.2010.04848.x. Epub 2010 Sep 30. Mol Ecol. 2010. PMID: 20887362
-
How does ecological disturbance influence genetic diversity?Trends Ecol Evol. 2013 Nov;28(11):670-9. doi: 10.1016/j.tree.2013.08.005. Epub 2013 Sep 19. Trends Ecol Evol. 2013. PMID: 24054910 Review.
-
Forest dynamics.F1000Res. 2016 Feb 17;5:F1000 Faculty Rev-183. doi: 10.12688/f1000research.7412.1. eCollection 2016. F1000Res. 2016. PMID: 26949526 Free PMC article. Review.
Cited by
-
Clonal reproduction as a driver of liana proliferation following large-scale disturbances in temperate forests.Am J Bot. 2025 Aug;112(8):e70085. doi: 10.1002/ajb2.70085. Epub 2025 Aug 13. Am J Bot. 2025. PMID: 40801659 Free PMC article.
-
Understanding how an amphicarpic species with a mixed mating system responds to fire: a population genetic approach.AoB Plants. 2021 Oct 21;13(6):plab067. doi: 10.1093/aobpla/plab067. eCollection 2021 Dec. AoB Plants. 2021. PMID: 34858568 Free PMC article.
-
Dispersal responses override density effects on genetic diversity during post-disturbance succession.Proc Biol Sci. 2016 Mar 30;283(1827):20152934. doi: 10.1098/rspb.2015.2934. Proc Biol Sci. 2016. PMID: 27009225 Free PMC article.
-
How Important Is Informed Natal Dispersal for Modelling the Demographic and Genetic Effects of Environmental Variability?Ecol Evol. 2024 Dec 16;14(12):e70681. doi: 10.1002/ece3.70681. eCollection 2024 Dec. Ecol Evol. 2024. PMID: 39691436 Free PMC article.
-
Resilience to periodic disturbances and the long-term genetic stability in Acropora coral.Commun Biol. 2024 Apr 4;7(1):410. doi: 10.1038/s42003-024-06100-0. Commun Biol. 2024. PMID: 38575730 Free PMC article.
References
-
- Amarasekare, P. , and Possingham H.. 2001. Patch dynamics and metapopulation theory: the case of successional species. J. Theor. Biol. 209:333–344. - PubMed
-
- Banks, S. C. , Dujardin M., McBurney L., Blair D., Barker M., and Lindenmayer D. B.. 2011. Starting points for small mammal population recovery after wildfire: recolonisation or residual populations? Oikos 120:26–37.
-
- Banks, S. C. , Cary G. J., Smith A. L., Davies I. D., Driscoll D. A., Gill A. M., et al. 2013. How does ecological disturbance influence genetic diversity? Trends Ecol. Evol. 28:670–679. - PubMed
-
- Banks, S. C. , Lorin T., Shaw R. E., McBurney L., Blair D., Blyton M. D., et al. 2015. Fine‐scale refuges can buffer demographic and genetic processes against short‐term climatic variation and disturbance: a 22‐year case study of an arboreal marsupial. Mol. Ecol. 24:3831–3845. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

