Lipidomic Analysis of Endocannabinoid Signaling: Targeted Metabolite Identification and Quantification
- PMID: 26839710
- PMCID: PMC4709765
- DOI: 10.1155/2016/2426398
Lipidomic Analysis of Endocannabinoid Signaling: Targeted Metabolite Identification and Quantification
Abstract
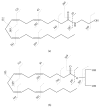
The endocannabinoids N-arachidonoylethanolamide (or anandamide, AEA) and 2-arachidonoylglycerol (2-AG) belong to the larger groups of N-acylethanolamines (NAEs) and monoacylglycerol (MAG) lipid classes, respectively. They are biologically active lipid molecules that activate G-protein-coupled cannabinoid receptors found in various organisms. After AEA and 2-AG were discovered in the 1990s, they have been extensively documented to have a broad range of physiological functions. Along with AEA, several NAEs, for example, N-palmitoylethanolamine (PEA), N-stearoylethanolamine (SEA), and N-oleoylethanolamine (OEA) are also present in tissues, usually at much larger concentrations than AEA. Any perturbation that involves the endocannabinoid pathway may subsequently alter basal level or metabolism of these lipid mediators. Further, the altered levels of these molecules often reflect pathological conditions associated with tissue damage. Robust and sensitive methodologies to analyze these lipid mediators are essential to understanding how they act as endocannabinoids. The recent advances in mass spectrometry allow researchers to develop lipidomics approaches and several methodologies have been proposed to quantify endocannabinoids in various biological systems.
Figures
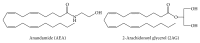

Similar articles
-
Troubleshooting in LC-MS/MS method for determining endocannabinoid and endocannabinoid-like molecules in rat brain structures applied to assessing the brain endocannabinoid/endovanilloid system significance.Toxicol Mech Methods. 2014 May;24(4):315-22. doi: 10.3109/15376516.2014.898356. Toxicol Mech Methods. 2014. PMID: 24576199
-
Comparative effects of parathion and chlorpyrifos on endocannabinoid and endocannabinoid-like lipid metabolites in rat striatum.Neurotoxicology. 2015 Sep;50:20-7. doi: 10.1016/j.neuro.2015.07.006. Epub 2015 Jul 26. Neurotoxicology. 2015. PMID: 26215119 Free PMC article.
-
Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis.Liver Int. 2010 Jul;30(6):816-25. doi: 10.1111/j.1478-3231.2009.02137.x. Epub 2009 Oct 14. Liver Int. 2010. PMID: 19840245
-
Proteomics and Lipidomics in the Elucidation of Endocannabinoid Signaling in Healthy and Schizophrenia Brains.Proteomics. 2018 Sep;18(18):e1700270. doi: 10.1002/pmic.201700270. Epub 2018 Aug 20. Proteomics. 2018. PMID: 30070429 Review.
-
From endocannabinoid profiling to 'endocannabinoid therapeutics'.Curr Opin Chem Biol. 2009 Jun;13(3):321-31. doi: 10.1016/j.cbpa.2009.04.615. Epub 2009 Jun 3. Curr Opin Chem Biol. 2009. PMID: 19497779 Review.
Cited by
-
Enhanced seedling growth by 3-n-pentadecylphenolethanolamide is mediated by fatty acid amide hydrolases in upland cotton (Gossypium hirsutum L.).Plant Direct. 2022 Jul 12;6(7):e421. doi: 10.1002/pld3.421. eCollection 2022 Jul. Plant Direct. 2022. PMID: 35844778 Free PMC article.
-
Fatty acid amide hydrolase and 9-lipoxygenase modulate cotton seedling growth by ethanolamide oxylipin levels.Plant Physiol. 2023 Feb 12;191(2):1234-1253. doi: 10.1093/plphys/kiac556. Plant Physiol. 2023. PMID: 36472510 Free PMC article.
-
Space and Time Coherent Mapping for Subcellular Resolution of Imaging Mass Spectrometry.Cells. 2022 Oct 26;11(21):3382. doi: 10.3390/cells11213382. Cells. 2022. PMID: 36359779 Free PMC article.
-
Endocannabinod Signal Dysregulation in Autism Spectrum Disorders: A Correlation Link between Inflammatory State and Neuro-Immune Alterations.Int J Mol Sci. 2017 Jul 3;18(7):1425. doi: 10.3390/ijms18071425. Int J Mol Sci. 2017. PMID: 28671614 Free PMC article. Review.
-
Simultaneous Assessment of Serum Levels and Pharmacologic Effects of Cannabinoids on Endocannabinoids and N-Acylethanolamines by Liquid Chromatography-Tandem Mass Spectrometry.Cannabis Cannabinoid Res. 2023 Aug;8(4):657-669. doi: 10.1089/can.2021.0181. Epub 2022 May 17. Cannabis Cannabinoid Res. 2023. PMID: 35580134 Free PMC article.
References
-
- Devane W. A., Dysarz F. A., Johnson M. R., Melvin L. S., Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology. 1988;34(5):605–613. - PubMed
-
- Matias I., Wang J. W., Moriello A. S., Nieves A., Woodward D. F., Di Marzo V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;75(6):413–418. doi: 10.1016/j.plefa.2006.08.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

