Isolation, Identification, and Characterisation of Degradation Products and the Development and Validation of a Stability-Indicating Method for the Estimation of Impurities in the Tolterodine Tartrate Formulation
- PMID: 26839802
- PMCID: PMC4727816
- DOI: 10.3797/scipharm.1407-18
Isolation, Identification, and Characterisation of Degradation Products and the Development and Validation of a Stability-Indicating Method for the Estimation of Impurities in the Tolterodine Tartrate Formulation
Abstract
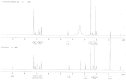
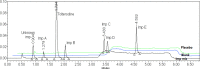
A short and sensitive stability-indicating gradient RP-UPLC method was developed for the quantitative determination of process-related impurities and degradation products of tolterodine tartrate in pharmaceutical formulations. The method was developed by using the Waters ACQUITY UPLC™ BEH shield RP18 (2.1 × 100 mm, 1.7 μm) column with a mobile phase containing a gradient mixture of solvent A and B at a detection wavelength of 210 nm. During the stress study, the degradation products of tolterodine tartrate were well-resolved from tolterodine and its impurities and the mass balances were found to be satisfactory in all the stress conditions, thus proving the stability-indicating capability of the method. The developed method was validated as per ICH guidelines with respect to specificity, linearity, limit of detection and quantification, accuracy, precision, ruggedness, and robustness. During the stability (40°C/75% RH, 3 months) analysis of the drug product, one unknown impurity was detected by the above stability-indicating method. The unknown impurity was isolated by preparative HPLC and subjected to mass and NMR studies. Based on the spectral data, the unknown impurity was characterised as 2-(3-amino-1-phenylpropyl)-4-methylphenol (des-N,N-diisopropyl tolterodine). Structural elucidation of the impurity by spectral data is discussed in detail.
Keywords: Characterisation; Development; Stability-indicating; Tolterodine tartrate; UPLC.
Figures


Similar articles
-
Identification and Characterization of an Oxidative Degradation Product of Fexofenadine, Development and Validation of a Stability-Indicating RP-UPLC Method for the Estimation of Process Related Impurities and Degradation Products of Fexofenadine in Pharmaceutical Formulations.Sci Pharm. 2012 Apr-Jun;80(2):295-309. doi: 10.3797/scipharm.1111-07. Epub 2012 Jan 21. Sci Pharm. 2012. PMID: 22896817 Free PMC article.
-
Development and Validation of a Stability-Indicating RP-HPLC Method for the Determination of Process-Related Impurities and Degradation Products of Rabeprazole Sodium in Pharmaceutical Formulation.Sci Pharm. 2013 Mar 17;81(3):697-711. doi: 10.3797/scipharm.1301-25. Print 2013 Jul-Sep. Sci Pharm. 2013. PMID: 24106668 Free PMC article.
-
Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream.J Chromatogr Sci. 2015 Jan;53(1):112-21. doi: 10.1093/chromsci/bmu027. Epub 2014 May 2. J Chromatogr Sci. 2015. PMID: 24795078
-
Choices of chromatographic methods as stability indicating assays for pharmaceutical products: A review.Heliyon. 2021 Mar 27;7(3):e06553. doi: 10.1016/j.heliyon.2021.e06553. eCollection 2021 Mar. Heliyon. 2021. PMID: 33855234 Free PMC article. Review.
-
Development of forced degradation and stability indicating studies of drugs-A review.J Pharm Anal. 2014 Jun;4(3):159-165. doi: 10.1016/j.jpha.2013.09.003. Epub 2013 Sep 17. J Pharm Anal. 2014. PMID: 29403878 Free PMC article. Review.
Cited by
-
HSPiP, Computational, and Thermodynamic Model-Based Optimized Solvents for Subcutaneous Delivery of Tolterodine Tartrate and GastroPlus-Based In Vivo Prediction in Humans: Part I.AAPS PharmSciTech. 2024 May 1;25(5):93. doi: 10.1208/s12249-024-02800-2. AAPS PharmSciTech. 2024. PMID: 38693316
References
-
- Drugs.com, Tolterodine tartrate. [(accessed17 July 2013)]. http://www.drugs.com/monograph/tolterodine-tartrate.html .
-
- Rxlist, Tolterodine tartrate. [(accessed17 July 2013)]. http://www.rxlist.com/detrol-la-drug.html .
-
- Drug Bank, Tolterodine tartrate. [(accessed17 July 2013)]. http://www.drugbank.ca/drugs/DB01036 .
-
- Nanda RK, Gaikwad J, Prakash A. Simultaneous spectrophotometric Estimation of Tolterodine on Pharmaceutical Dosage form. Int J PharmTechRes. 2009;2:312–314.
-
- Nanda RK, Gaikwad J, Prakash A. Estimation of Tamsulosin and Tolterodine in its pharmaceutical dosage form by Spectrophotometric method. Int J PharmTechRes. 2009;1:420–423.
LinkOut - more resources
Full Text Sources
Other Literature Sources
