Simultaneous Determination and Pharmacokinetic Study of Losartan, Losartan Carboxylic Acid, Ramipril, Ramiprilat, and Hydrochlorothiazide in Rat Plasma by a Liquid Chromatography/Tandem Mass Spectrometry Method
- PMID: 26839805
- PMCID: PMC4729183
- DOI: 10.3797/scipharm.1410-15
Simultaneous Determination and Pharmacokinetic Study of Losartan, Losartan Carboxylic Acid, Ramipril, Ramiprilat, and Hydrochlorothiazide in Rat Plasma by a Liquid Chromatography/Tandem Mass Spectrometry Method
Abstract
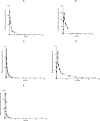
The monitoring of the plasmatic concentrations of cardiovascular drugs is crucial for understanding their pharmacokinetics and pharmacodynamics. A simple, sensitive, specific, and high-throughput liquid chromatography/tandem mass spectrometry (LC-MS/MS) method was developed and validated for the simultaneous estimation and pharmacokinetic study of losartan (LOS), losartan carboxylic acid (LCA), ramipril (RAM), ramiprilate (RPT), and hydrochlorothiazide (HCZ) in rat plasma using irbesartan (IBS) and metolazone (MET) as internal standards (ISs). After solid phase extraction (SPE), analytes and ISs were separated on an Agilent Poroshell 120, EC-C18 (50 mm × 4.6 mm, i.d., 2.7 μm) column with a mobile phase consisting of methanol/water (85:15, v/v) containing 5 mmol/L ammonium formate and 0.1% formic acid at a flow rate of 0.4 mL/min. The precursor → product ion transitions for the analytes and ISs were monitored on a triple quadrupole mass spectrometer, operating in the multiple reaction monitoring (MRM) mode and switching the electrospray ionization (ESI) mode during chromatography from positive (to detect LOS, LCA, RAM, RPT, and IBS) to negative (to detect HCZ and MET). The method was validated as per the FDA guidelines and it exhibited sufficient specificity, accuracy, and precision. The method was found to be linear in the range of 3-3000 ng/mL for LOS and LCA, 0.1-200 ng/mL for RAM and RPT, and 1-1500 ng/mL for HCZ. The described method was successfully applied to the preclinical pharmacokinetic study of analytes after oral administration of a mixture of LOS (10 mg/kg), RAM (1 mg/kg), and HCZ (2.5 mg/kg) in rats.
Keywords: LC-MS/MS; Pharmacokinetic; Plasma; Simultaneous estimation; Validation.
Figures


Similar articles
-
Simultaneous Determination and Pharmacokinetics of Metolazone, Losartan and Losartan Carboxylic Acid in Rat Plasma by HPLC-ESI-MS-MS.J Chromatogr Sci. 2015 Oct;53(9):1520-7. doi: 10.1093/chromsci/bmv047. Epub 2015 May 6. J Chromatogr Sci. 2015. PMID: 25947361
-
Development and validation of bioanalytical method for simultaneous estimation of ramipril and hydrochlorothiazide in human plasma using liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Nov 1;970:53-9. doi: 10.1016/j.jchromb.2014.08.023. Epub 2014 Sep 6. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 25240204
-
Simultaneous determination of losartan, EXP-3174 and hydrochlorothiazide in plasma via fully automated 96-well-format-based solid-phase extraction and liquid chromatography-negative electrospray tandem mass spectrometry.Anal Bioanal Chem. 2007 Jan;387(2):593-601. doi: 10.1007/s00216-006-0990-4. Epub 2006 Nov 21. Anal Bioanal Chem. 2007. PMID: 17119933
-
Simultaneous Determination of Aliskiren Hemifumarate, Amlodipine Besylate and Hydrochlorothiazide in Spiked Human Plasma Using UPLC-MS/MS.J Chromatogr Sci. 2015 Aug;53(7):1178-84. doi: 10.1093/chromsci/bmu213. Epub 2015 Jan 9. J Chromatogr Sci. 2015. PMID: 25575509
-
Development and validation of an analytical method for trace-level quantification of genotoxic nitrosamine impurities in losartan and hydrochlorothiazide fixed-dose combination tablets using ultra performance liquid chromatography triple quadrupole mass spectrometry.Rapid Commun Mass Spectrom. 2023 Apr 30;37(8):e9488. doi: 10.1002/rcm.9488. Rapid Commun Mass Spectrom. 2023. PMID: 36740827 Review.
Cited by
-
A Rapid, Sensitive, and High-throughput Method for the Simultaneous Determination of Antihypertensive Drug Combinations in Dog Plasma by UHPLC-MS/MS: The Assessment of Predicable Bioequivalence of In-vitro Dissolution Condition.Curr Pharm Des. 2024;30(32):2574-2585. doi: 10.2174/0113816128295265240613061905. Curr Pharm Des. 2024. PMID: 38956914
-
Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence.Clin Pharmacol Ther. 2020 Aug;108(2):236-241. doi: 10.1002/cpt.1863. Epub 2020 May 10. Clin Pharmacol Ther. 2020. PMID: 32320478 Free PMC article. Review.
References
-
- Volpe M, Tocci G. Rationale for triple fixed-dose combination therapy with an angiotensin II receptor blocker, a calcium channel blocker, and a thiazide diuretic. Vasc Health Risk Manag. 2012;8:371–380. http://dx.doi.org/10.2147/VHRM.S28359 . - PMC - PubMed
-
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. http://dx.doi.org/10.1161/01.HYP.0000107251.49515.c2 . - PubMed
-
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Hypertension. 2013;31:1281–1357. http://dx.doi.org/0.1097/01.hjh.0000431740.32696.cc . - PubMed
-
- Gradman AH. Strategies for combination therapy in hypertension. Curr Opin Nephrol Hypertens. 2012;21:486–491. http://dx.doi.org/10.1097/MNH.0b013e328356c551 . - PubMed
-
- Channer KS, McLean KA, Lawson-Matthew, Richardson M. Combination diuretic treatment in severe heart failure: a randomised controlled trial. Br Heart J. 1994;71:146–150. http://dx.doi.org/10.1136/hrt.71.2.146 . - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
