Enhancing Skin Permeation of Biphenylacetic Acid (BPA) Using Salt Formation with Organic and Alkali Metal Bases
- PMID: 26839810
- PMCID: PMC4727820
- DOI: 10.3797/scipharm.1406-19
Enhancing Skin Permeation of Biphenylacetic Acid (BPA) Using Salt Formation with Organic and Alkali Metal Bases
Abstract
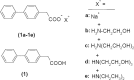
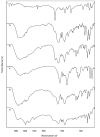
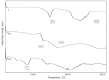
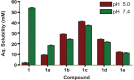
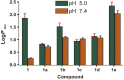
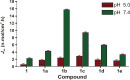
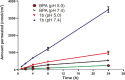
In the present study, a series of organic and alkali metal salts of biphenylacetic acid (BPA) have been prepared and evaluated in vitro for percutaneous drug delivery. The physicochemical properties of BPA salts were determined using solubility measurements, DSC, and IR. The DSC thermogram and FTIR spectra confirmed the salt formation with organic and alkali metal bases. Among the series, salts with organic amines (ethanolamine, diethanolamine, triethanolamine, and diethylamine) had lowered melting points while the alkali metal salt (sodium) had a higher melting point than BPA. The in vitro study showed that salt formation improves the physicochemical properties of BPA, leading to improved permeability through the skin. Amongst all the prepared salts, ethanolamine salt (1b) showed 7.2- and 5.4-fold higher skin permeation than the parent drug at pH 7.4 and 5.0, respectively, using rat skin.
Keywords: BPA; Lipophilicity; NSAID; Salt; Skin permeation.
Figures

Similar articles
-
Enhanced skin permeation of 6-methoxy-2-naphthylacetic acid by salt formation.Drug Deliv. 2015 May;22(3):359-66. doi: 10.3109/10717544.2014.894595. Epub 2014 Mar 27. Drug Deliv. 2015. PMID: 24670100
-
The effect of meloxicam/ethanolamine salt formation on percutaneous absorption of meloxicam.Arch Pharm Res. 2007 Feb;30(2):215-21. doi: 10.1007/BF02977697. Arch Pharm Res. 2007. PMID: 17366744
-
Enhanced percutaneous absoption of piroxicam via salt formation with ethanolamines.Pharm Res. 2002 Sep;19(9):1375-80. doi: 10.1023/a:1020367212307. Pharm Res. 2002. PMID: 12403076
-
Improved absorption of meloxicam via salt formation with ethanolamines.Eur J Pharm Biopharm. 2007 Jan;65(1):99-103. doi: 10.1016/j.ejpb.2006.07.003. Epub 2006 Jul 15. Eur J Pharm Biopharm. 2007. PMID: 16919925
-
Enhanced bioavailability of piroxicam via salt formation with ethanolamines.Int J Pharm. 2005 Jun 13;297(1-2):156-61. doi: 10.1016/j.ijpharm.2005.03.016. Epub 2005 Apr 25. Int J Pharm. 2005. PMID: 15907602
References
-
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. http://www.ncbi.nlm.nih.gov/pubmed/10508233 . - PubMed
-
- Johnson AG, Quinn DI, Day RO. Non-steroidal anti-inflammatory drugs. Med J Aust. 1995;163:155–158. http://www.ncbi.nlm.nih.gov/pubmed/7643770 . - PubMed
-
- Figueras A, Capellà D, Castel JM, Laorte JR. Spontaneous reporting of adverse drug reactions to non-stroidal anti-inflammatory drugs. A report from the spanish system of pharmacovigilance, including an early analysis of topical and enteric coated formulations. Eur J Clin Pharmacol. 1994;47:297–303. http://dx.doi.org/10.1007/BF00191158 . - PubMed
-
- Lichtenstein DR, Syngal S, Wolfe MM. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. The double edged sword. Arthritis Rheum. 1995;38:5–18. http://dx.doi.org/10.1002/art.1780380103 . - PubMed
-
- Hansen TM, Matzen P, Madsen P. Endoscopic evaluation of the effect of indomethacin capsules and suppositories on the gastric mucosa in rheumatic patients. J Rheumatol. 1984;11:484–487. http://www.ncbi.nlm.nih.gov/pubmed/6332910 . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
