Paeonia lactiflora Enhances the Adhesion of Trophoblast to the Endometrium via Induction of Leukemia Inhibitory Factor Expression
- PMID: 26839969
- PMCID: PMC4739624
- DOI: 10.1371/journal.pone.0148232
Paeonia lactiflora Enhances the Adhesion of Trophoblast to the Endometrium via Induction of Leukemia Inhibitory Factor Expression
Abstract
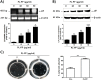
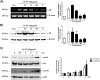
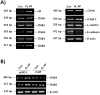
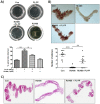
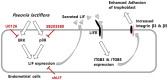
In the present study, we investigated the role of Paeonia lactiflora Pall. extract on embryo implantation in vitro and in vivo. A polysaccharides depleted-water extract of P. lactiflora (PL-PP) increased LIF expression in human endometrial Ishikawa cells at non-cytotoxic doses. PL-PP significantly increased the adhesion of the human trophectoderm-derived JAr spheroids to endometrial Ishikawa cells. PL-PP-induced LIF expression was decreased in the presence of a p38 kinase inhibitor SB203580 and an MEK/ERK inhibitor U0126. Furthermore, endometrial LIF knockdown by shRNA reduced the expression of integrins β3 and β5 and adhesion of JAr spheroids to Ishikawa cells. In vivo administration of PL-PP restored the implantation of mouse blastocysts in a mifepristone-induced implantation failure mice model. Our results demonstrate that PL-PP increases LIF expression via the p38 and MEK/ERK pathways and favors trophoblast adhesion to endometrial cells.
Conflict of interest statement
Figures


Similar articles
-
Water-extracted Perilla frutescens increases endometrial receptivity though leukemia inhibitory factor-dependent expression of integrins.J Pharmacol Sci. 2016 Aug;131(4):259-66. doi: 10.1016/j.jphs.2016.07.004. Epub 2016 Jul 25. J Pharmacol Sci. 2016. PMID: 27562703
-
Water-extracted tubers of Cyperus rotundus L. enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin αVβ3 and αVβ5.J Ethnopharmacol. 2017 Aug 17;208:16-23. doi: 10.1016/j.jep.2017.06.051. Epub 2017 Jul 1. J Ethnopharmacol. 2017. PMID: 28676452
-
Extracts from Paeonia lactiflora Pallas, Rehmannia Glutinosa var. Purpurea Makino, Perilla Frutescens var. Acuta Kudo may increase the endometrial receptivity through expression of leukemia inhibitory factor and adhesion molecules.J Tradit Chin Med. 2019 Feb;39(1):15-25. J Tradit Chin Med. 2019. PMID: 32186019
-
Review: LIF and IL11 in trophoblast-endometrial interactions during the establishment of pregnancy.Placenta. 2010 Mar;31 Suppl:S99-104. doi: 10.1016/j.placenta.2009.12.027. Epub 2010 Feb 2. Placenta. 2010. PMID: 20129664 Review.
-
Leukemia inhibitory factor: roles in embryo implantation and in nonhormonal contraception.ScientificWorldJournal. 2014;2014:201514. doi: 10.1155/2014/201514. Epub 2014 Jul 24. ScientificWorldJournal. 2014. PMID: 25152902 Free PMC article. Review.
Cited by
-
Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation.Pharmaceuticals (Basel). 2021 Dec 31;15(1):53. doi: 10.3390/ph15010053. Pharmaceuticals (Basel). 2021. PMID: 35056110 Free PMC article. Review.
-
Paeoniflorin Enhances Endometrial Receptivity through Leukemia Inhibitory Factor.Biomolecules. 2021 Mar 16;11(3):439. doi: 10.3390/biom11030439. Biomolecules. 2021. PMID: 33809755 Free PMC article.
-
Administration of a herbal formulation enhanced blastocyst implantation via IκB activation in mouse endometrium.Chin Med. 2020 Oct 20;15:112. doi: 10.1186/s13020-020-00395-x. eCollection 2020. Chin Med. 2020. PMID: 33093859 Free PMC article.
-
Psoralen Promotes Proliferation, Migration, and Invasion of Human Extravillous Trophoblast Derived HTR-8/Svneo Cells in vitro by NF-κB Pathway.Front Pharmacol. 2022 Apr 8;13:804400. doi: 10.3389/fphar.2022.804400. eCollection 2022. Front Pharmacol. 2022. PMID: 35462898 Free PMC article.
-
Restoration of miR-223-3p expression in aged mouse uteri with Samul-tang administration.Integr Med Res. 2022 Jun;11(2):100835. doi: 10.1016/j.imr.2022.100835. Epub 2022 Jan 24. Integr Med Res. 2022. PMID: 35141134 Free PMC article.
References
-
- Diedrich K, Fauser BC, Devroey P, Griesinger G (2007) The role of the endometrium and embryo in human implantation. Hum Reprod Update 13: 365–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

