Competitive Mirror Image Phage Display Derived Peptide Modulates Amyloid Beta Aggregation and Toxicity
- PMID: 26840229
- PMCID: PMC4740492
- DOI: 10.1371/journal.pone.0147470
Competitive Mirror Image Phage Display Derived Peptide Modulates Amyloid Beta Aggregation and Toxicity
Erratum in
-
Correction: Competitive Mirror Image Phage Display Derived Peptide Modulates Amyloid Beta Aggregation and Toxicity.PLoS One. 2016 Jul 12;11(7):e0159470. doi: 10.1371/journal.pone.0159470. eCollection 2016. PLoS One. 2016. PMID: 27403661 Free PMC article.
Abstract
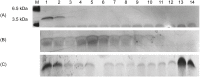
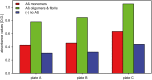
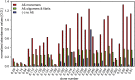
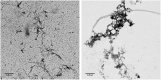
Alzheimer´s disease is the most prominent type of dementia and currently no causative treatment is available. According to recent studies, oligomeric species of the amyloid beta (Aβ) peptide appear to be the most toxic Aβ assemblies. Aβ monomers, however, may be not toxic per se and may even have a neuroprotective role. Here we describe a competitive mirror image phage display procedure that allowed us to identify preferentially Aβ1-42 monomer binding and thereby stabilizing peptides, which destabilize and thereby eliminate toxic oligomer species. One of the peptides, called Mosd1 (monomer specific d-peptide 1), was characterized in more detail. Mosd1 abolished oligomers from a mixture of Aβ1-42 species, reduced Aβ1-42 toxicity in cell culture, and restored the physiological phenotype in neuronal cells stably transfected with the gene coding for human amyloid precursor protein.
Conflict of interest statement
Figures


Similar articles
-
A cyclic KLVFF-derived peptide aggregation inhibitor induces the formation of less-toxic off-pathway amyloid-β oligomers.Chembiochem. 2014 Nov 24;15(17):2577-83. doi: 10.1002/cbic.201402430. Epub 2014 Sep 26. Chembiochem. 2014. PMID: 25262917
-
Curcumin Attenuates Amyloid-β Aggregate Toxicity and Modulates Amyloid-β Aggregation Pathway.ACS Chem Neurosci. 2016 Jan 20;7(1):56-68. doi: 10.1021/acschemneuro.5b00214. Epub 2015 Nov 17. ACS Chem Neurosci. 2016. PMID: 26529184
-
Covalent modifier-type aggregation inhibitor of amyloid-β based on a cyclo-KLVFF motif.Bioorg Med Chem Lett. 2015 Aug 1;25(15):2972-5. doi: 10.1016/j.bmcl.2015.05.027. Epub 2015 May 19. Bioorg Med Chem Lett. 2015. PMID: 26045033
-
Medicinal Chemistry Focusing on Aggregation of Amyloid-β.Chem Pharm Bull (Tokyo). 2016;64(1):1-7. doi: 10.1248/cpb.c15-00742. Chem Pharm Bull (Tokyo). 2016. PMID: 26726739 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
A screened PirB antagonist peptide antagonizes Aβ42-mediated inhibition of neurite outgrowth in vitro.Appl Microbiol Biotechnol. 2021 Jun;105(11):4649-4662. doi: 10.1007/s00253-021-11363-2. Epub 2021 Jun 1. Appl Microbiol Biotechnol. 2021. PMID: 34059940
-
Kaempferia parviflora extract and its methoxyflavones as potential anti-Alzheimer assessing in vitro, integrated computational approach, and in vivo impact on behaviour in scopolamine-induced amnesic mice.PLoS One. 2025 Mar 10;20(3):e0316888. doi: 10.1371/journal.pone.0316888. eCollection 2025. PLoS One. 2025. PMID: 40063637 Free PMC article.
-
Trends in peptide drug discovery.Nat Rev Drug Discov. 2021 Apr;20(4):309-325. doi: 10.1038/s41573-020-00135-8. Epub 2021 Feb 3. Nat Rev Drug Discov. 2021. PMID: 33536635
-
Phage Display Technology in Biomarker Identification with Emphasis on Non-Cancerous Diseases.Molecules. 2024 Jun 25;29(13):3002. doi: 10.3390/molecules29133002. Molecules. 2024. PMID: 38998954 Free PMC article. Review.
-
D-Peptide and D-Protein Technology: Recent Advances, Challenges, and Opportunities.Chembiochem. 2023 Feb 14;24(4):e202200537. doi: 10.1002/cbic.202200537. Epub 2022 Nov 16. Chembiochem. 2023. PMID: 36278392 Free PMC article. Review.
References
-
- Organization WH. Dementia: A Public Health Priority: World Health Organization; 2012.
-
- Ritchie K, Lovestone S. The dementias. Lancet. 2002;360(9347):1759–66. Epub 2002/12/14. . - PubMed
-
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399(6738 Suppl):A23–31. Epub 1999/07/07. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

