Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres
- PMID: 26840303
- PMCID: PMC4783918
- DOI: 10.3390/ijms17020184
Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres
Abstract
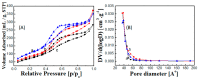
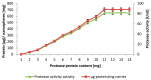
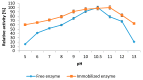
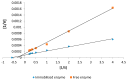
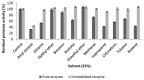
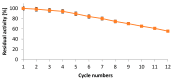
The stability and reusability of soluble enzymes are of major concerns, which limit their industrial applications. Herein, alkaline protease from Bacillus sp. NPST-AK15 was immobilized onto hollow core-mesoporous shell silica (HCMSS) nanospheres. Subsequently, the properties of immobilized proteases were evaluated. Non-, ethane- and amino-functionalized HCMSS nanospheres were synthesized and characterized. NPST-AK15 was immobilized onto the synthesized nano-supports by physical and covalent immobilization approaches. However, protease immobilization by covalent attachment onto the activated HCMSS-NH₂ nanospheres showed highest immobilization yield (75.6%) and loading capacity (88.1 μg protein/mg carrier) and was applied in the further studies. In comparison to free enzyme, the covalently immobilized protease exhibited a slight shift in the optimal pH from 10.5 to 11.0, respectively. The optimum temperature for catalytic activity of both free and immobilized enzyme was seen at 60 °C. However, while the free enzyme was completely inactivated when treated at 60 °C for 1 h the immobilized enzyme still retained 63.6% of its initial activity. The immobilized protease showed higher V(max), k(cat) and k(cat)/K(m), than soluble enzyme by 1.6-, 1.6- and 2.4-fold, respectively. In addition, the immobilized protease affinity to the substrate increased by about 1.5-fold. Furthermore, the enzyme stability in various organic solvents was significantly enhanced upon immobilization. Interestingly, the immobilized enzyme exhibited much higher stability in several commercial detergents including OMO, Tide, Ariel, Bonux and Xra by up to 5.2-fold. Finally, the immobilized protease maintained significant catalytic efficiency for twelve consecutive reaction cycles. These results suggest the effectiveness of the developed nanobiocatalyst as a candidate for detergent formulation and peptide synthesis in non-aqueous media.
Keywords: alkaline protease; alkaliphiles; detergents; hollow core-mesoporous shell silica nanospheres; immobilization; nanotechnology.
Figures






Similar articles
-
Development of novel robust nanobiocatalyst for detergents formulations and the other applications of alkaline protease.Bioprocess Biosyst Eng. 2016 May;39(5):793-805. doi: 10.1007/s00449-016-1559-z. Epub 2016 Feb 10. Bioprocess Biosyst Eng. 2016. PMID: 26861651
-
Stabilization and improved properties of Salipaludibacillus agaradhaerens alkaline protease by immobilization onto double mesoporous core-shell nanospheres.Int J Biol Macromol. 2021 Jan 1;166:557-566. doi: 10.1016/j.ijbiomac.2020.10.213. Epub 2020 Nov 10. Int J Biol Macromol. 2021. PMID: 33186653
-
Enzyme shielding by mesoporous organosilica shell on Fe3O4@silica yolk-shell nanospheres.Int J Biol Macromol. 2018 Oct 1;117:673-682. doi: 10.1016/j.ijbiomac.2018.05.227. Epub 2018 May 31. Int J Biol Macromol. 2018. PMID: 29859841
-
Enzymes immobilized in mesoporous silica: a physical-chemical perspective.Adv Colloid Interface Sci. 2014 Mar;205:339-60. doi: 10.1016/j.cis.2013.08.010. Epub 2013 Sep 10. Adv Colloid Interface Sci. 2014. PMID: 24112562 Review.
-
Nanocarriers Immobilized Proteases and Their Industrial Applications: An Overview.J Nanosci Nanotechnol. 2018 Jan 1;18(1):486-499. doi: 10.1166/jnn.2018.15246. J Nanosci Nanotechnol. 2018. PMID: 29768874 Review.
Cited by
-
Catalytic, kinetic and thermodynamic properties of free and immobilized caseinase on mica glass-ceramics.Heliyon. 2019 May 9;5(5):e01674. doi: 10.1016/j.heliyon.2019.e01674. eCollection 2019 May. Heliyon. 2019. PMID: 31193050 Free PMC article.
-
Enzyme Immobilization Technologies and Industrial Applications.ACS Omega. 2023 Jan 31;8(6):5184-5196. doi: 10.1021/acsomega.2c07560. eCollection 2023 Feb 14. ACS Omega. 2023. PMID: 36816672 Free PMC article. Review.
-
Grafted carrageenan: alginate gel beads for catalase enzyme covalent immobilization.3 Biotech. 2021 Jul;11(7):341. doi: 10.1007/s13205-021-02875-9. Epub 2021 Jun 16. 3 Biotech. 2021. PMID: 34221812 Free PMC article.
-
Immobilization of the Highly Active UDP-Glucose Pyrophosphorylase From Thermocrispum agreste Provides a Highly Efficient Biocatalyst for the Production of UDP-Glucose.Front Bioeng Biotechnol. 2020 Jul 2;8:740. doi: 10.3389/fbioe.2020.00740. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32714915 Free PMC article.
-
Alcalase Microarray Base on Metal Ion Modified Hollow Mesoporous Silica Spheres as a Sustainable and Efficient Catalysis Platform for Proteolysis.Front Bioeng Biotechnol. 2020 Jun 10;8:565. doi: 10.3389/fbioe.2020.00565. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32587851 Free PMC article.
References
-
- Jain D., Pancha I., Mishra S.K., Shrivastav A., Mishra S. Purification and characterization of haloalkaline thermoactive, solvent stable and SDS-induced protease from Bacillus sp.: A potential additive for laundry detergents. Bioresour. Technol. 2012;115:228–236. doi: 10.1016/j.biortech.2011.10.081. - DOI - PubMed
-
- Soozanipour A., Taheri-Kafrani A., Isfahani A.L. Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2015;270:235–243. doi: 10.1016/j.cej.2015.02.032. - DOI
-
- Bouacem K., Bouanane-Darenfed A., Laribi-Habchi H., ben Elhoul M., Hmida-Sayari A., Hacene H., Ollivier B., Fardeau M.L., Jaouadi B., Bejar S. Biochemical characterization of a detergent stable serine alkalineprotease from Caldicoprobacter guelmensis. Int. J. Biol. Macromol. 2015;81:299–307. doi: 10.1016/j.ijbiomac.2015.08.011. - DOI - PubMed
-
- Ben Elhoul M., Jaouadi N.Z., Rekik H., Bejar W., Touioui S.B., Hmidi M., Badis A., Bejar S., Jaouadi B. A novel detergent-stable solvent-tolerant serine thiol alkaline protease from Streptomyces koyangensis TN650. Int. J. Biol. Macromol. 2015;79:871–882. doi: 10.1016/j.ijbiomac.2015.06.006. - DOI - PubMed
-
- Patil U., Chaudhari A. Purification and characterizationofsolvent-tolerant, thermostable, alkaline metalloprotease from alkalophilic Pseudomonas aeruginosa MTCC 7926. J. Chem. Technol. Biotechnol. 2009;84:1255–1262. doi: 10.1002/jctb.2169. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

