Systematic Review of Inhaled Bronchodilator and Corticosteroid Therapies in Infants with Bronchopulmonary Dysplasia: Implications and Future Directions
- PMID: 26840339
- PMCID: PMC4740433
- DOI: 10.1371/journal.pone.0148188
Systematic Review of Inhaled Bronchodilator and Corticosteroid Therapies in Infants with Bronchopulmonary Dysplasia: Implications and Future Directions
Abstract
Background: There is much debate surrounding the use of inhaled bronchodilators and corticosteroids for infants with bronchopulmonary dysplasia (BPD).
Objective: The objective of this systematic review was to identify strengths and knowledge gaps in the literature regarding inhaled therapies in BPD and guide future research to improve long-termoutcomes.
Methods: The databases of Academic Search Complete, CINAHL, PUBMED/MEDLINE, and Scopus were searched for studies that evaluated both acute and long-term clinical outcomes related to the delivery and therapeutic efficacy of inhaled beta-agonists, anticholinergics and corticosteroids in infants with developing and/or established BPD.
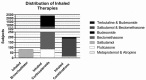
Results: Of 181 articles, 22 met inclusion criteria for review. Five evaluated beta-agonist therapies (n = 84, weighted gestational age (GA) of 27.1(26-30) weeks, weighted birth weight (BW) of 974(843-1310) grams, weighted post menstrual age (PMA) of 34.8(28-39) weeks, and weighted age of 53(15-86) days old at the time of evaluation). Fourteen evaluated inhaled corticosteroids (n = 2383, GA 26.2(26-29) weeks, weighted BW of 853(760-1114) grams, weighted PMA of 27.0(26-31) weeks, and weighted age of 6(0-45) days old at time of evaluation). Three evaluated combination therapies (n = 198, weighted GA of 27.8(27-29) weeks, weighted BW of 1057(898-1247) grams, weighted PMA of 30.7(29-45) weeks, and age 20(10-111) days old at time of evaluation).
Conclusion: Whether inhaled bronchodilators and inhaled corticosteroids improve long-term outcomes in BPD remains unclear. Literature regarding these therapies mostly addresses evolving BPD. There appears to be heterogeneity in treatment responses, and may be related to varying modes of administration. Further research is needed to evaluate inhaled therapies in infants with severe BPD. Such investigations should focus on appropriate definitions of disease and subject selection, timing of therapies, and new drugs, devices and delivery methods as compared to traditional methods across all modalities of respiratory support, in addition to the assessment of long-term outcomes of initial responders.
Conflict of interest statement
Figures
References
-
- Tin W, Wiswell TE. Adjunctive therapies in chronic lung disease: examining the evidence. Semin Fetal Neonatal Med. 2008;13(1):44–52. Epub 2007/11/07. - PubMed
-
- Bancalari E, Wilson-Costello D, Iben SC. Management of infants with bronchopulmonary dysplasia in North America. Early Hum Dev. 2005;81(2):171–9. Epub 2005/03/08. - PubMed
-
- Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. ATS statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168(3):356–96. Epub 2003/07/31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous