Identification of Human Islet Amyloid Polypeptide as a BACE2 Substrate
- PMID: 26840340
- PMCID: PMC4739698
- DOI: 10.1371/journal.pone.0147254
Identification of Human Islet Amyloid Polypeptide as a BACE2 Substrate
Abstract
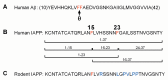
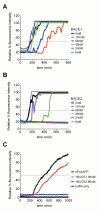
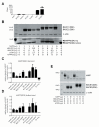
Pancreatic amyloid formation by islet amyloid polypeptide (IAPP) is a hallmark pathological feature of type 2 diabetes. IAPP is stored in the secretory granules of pancreatic beta-cells and co-secreted with insulin to maintain glucose homeostasis. IAPP is innocuous under homeostatic conditions but imbalances in production or processing of IAPP may result in homodimer formation leading to the rapid production of cytotoxic oligomers and amyloid fibrils. The consequence is beta-cell dysfunction and the accumulation of proteinaceous plaques in and around pancreatic islets. Beta-site APP-cleaving enzyme 2, BACE2, is an aspartyl protease commonly associated with BACE1, a related homolog responsible for amyloid processing in the brain and strongly implicated in Alzheimer's disease. Herein, we identify two distinct sites of the mature human IAPP sequence that are susceptible to BACE2-mediated proteolytic activity. The result of proteolysis is modulation of human IAPP fibrillation and human IAPP protein degradation. These results suggest a potential therapeutic role for BACE2 in type 2 diabetes-associated hyperamylinaemia.
Conflict of interest statement
Figures





Similar articles
-
BACE2 suppression promotes β-cell survival and function in a model of type 2 diabetes induced by human islet amyloid polypeptide overexpression.Cell Mol Life Sci. 2017 Aug;74(15):2827-2838. doi: 10.1007/s00018-017-2505-1. Epub 2017 Mar 23. Cell Mol Life Sci. 2017. PMID: 28337562 Free PMC article.
-
Inhibition of BACE2 counteracts hIAPP-induced insulin secretory defects in pancreatic β-cells.FASEB J. 2015 Jan;29(1):95-104. doi: 10.1096/fj.14-255489. Epub 2014 Oct 23. FASEB J. 2015. PMID: 25342134
-
Islet amyloid polypeptide in pancreatic islets from type 2 diabetic subjects.Islets. 2012 May-Jun;4(3):223-32. doi: 10.4161/isl.20477. Islets. 2012. PMID: 22847497 Free PMC article.
-
Islet amyloid polypeptide, islet amyloid, and diabetes mellitus.Physiol Rev. 2011 Jul;91(3):795-826. doi: 10.1152/physrev.00042.2009. Physiol Rev. 2011. PMID: 21742788 Review.
-
The β-cell assassin: IAPP cytotoxicity.J Mol Endocrinol. 2017 Oct;59(3):R121-R140. doi: 10.1530/JME-17-0105. Epub 2017 Aug 15. J Mol Endocrinol. 2017. PMID: 28811318 Review.
Cited by
-
Inside the Insulin Secretory Granule.Metabolites. 2021 Aug 5;11(8):515. doi: 10.3390/metabo11080515. Metabolites. 2021. PMID: 34436456 Free PMC article. Review.
-
Physiological Functions of the β-Site Amyloid Precursor Protein Cleaving Enzyme 1 and 2.Front Mol Neurosci. 2017 Apr 19;10:97. doi: 10.3389/fnmol.2017.00097. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28469554 Free PMC article. Review.
-
The β-Secretase BACE1 in Alzheimer's Disease.Biol Psychiatry. 2021 Apr 15;89(8):745-756. doi: 10.1016/j.biopsych.2020.02.001. Epub 2020 Feb 13. Biol Psychiatry. 2021. PMID: 32223911 Free PMC article. Review.
-
Biological Mechanism-based Neurology and Psychiatry: A BACE1/2 and Downstream Pathway Model.Curr Neuropharmacol. 2023;21(1):31-53. doi: 10.2174/1570159X19666211201095701. Curr Neuropharmacol. 2023. PMID: 34852743 Free PMC article.
-
The emerging role of β-secretases in cancer.J Exp Clin Cancer Res. 2021 Apr 29;40(1):147. doi: 10.1186/s13046-021-01953-3. J Exp Clin Cancer Res. 2021. PMID: 33926496 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

