Disorganization of the splenic microanatomy in ageing mice
- PMID: 26840375
- PMCID: PMC4819137
- DOI: 10.1111/imm.12590
Disorganization of the splenic microanatomy in ageing mice
Abstract
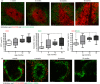
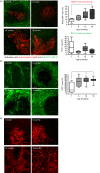
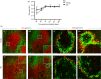
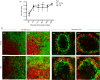
The precise mechanisms responsible for immunosenescence still remain to be determined, however, considering the evidence that disruption of the organization of primary and secondary lymphoid organs results in immunodeficiency, we propose that this could be involved in the decline of immune responses with age. Therefore, we investigated the integrity of the splenic microarchitecture in mice of increasing age and its reorganization following immune challenge in young and old mice. Several differences in the anatomy of the spleen with age in both the immune and stromal cells were observed. There is an age-related increase in the overall size of the white pulp, which occurs primarily within the T-cell zone and is mirrored by the enlargement of the T-cell stromal area, concurrent to the distinct boundary between T cells and B cells becoming less defined in older mice. In conjunction, there appears to be a loss of marginal zone macrophages, which is accompanied by an accumulation of fibroblasts in the spleens from older animals. Furthermore, whereas the reorganization of the white pulp is resolved after several days following antigenic challenge in young animals, it remains perturbed in older subjects. All these age-related changes within the spleen could potentially contribute to the age-dependent deficiencies in functional immunity.
Keywords: rodent; spleen and lymph nodes; stromal cells.
© 2016 John Wiley & Sons Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

