Expression of Streptococcus pneumoniae Bacteriocins Is Induced by Antibiotics via Regulatory Interplay with the Competence System
- PMID: 26840404
- PMCID: PMC4739728
- DOI: 10.1371/journal.ppat.1005422
Expression of Streptococcus pneumoniae Bacteriocins Is Induced by Antibiotics via Regulatory Interplay with the Competence System
Abstract
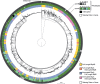
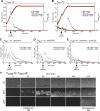
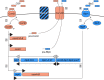
Pneumococcal bacteriocins (pneumocins) are antibacterial toxins that mediate intra-species competition within the human host. However, the triggers of pneumocin expression are poorly understood. Using RNA-sequencing, we mapped the regulon of the pneumocin cluster (blp) of Streptococcus pneumoniae D39. Furthermore, by analogy with pneumococcal competence, we show that several antibiotics activate the blp-genes. Using real-time gene expression measurements we show that while the promoter driving expression of the two-component regulatory system blpR/H is constitutive, the remaining blp-promoters that control pneumocin expression, immunity and the inducer peptide BlpC, are pH-dependent and induced in the late exponential phase. Intriguingly, competence for genetic transformation, mediated by the paralogous ComD/E two-component quorum system, is induced by the same environmental cues. To test for interplay between these regulatory systems, we quantified the regulatory response to the addition of synthetic BlpC and competence-stimulating peptide (CSP). Supporting the idea of such interplay, we found that immediately upon addition of CSP, the blp-promoters were activated in a comD/E-dependent manner. After a delay, blp-expression was highly induced and was strictly dependent on blpRH and blpC. This raised the question of the mechanism of BlpC export, since bioinformatic analysis showed that the genes encoding the putative exporter for BlpC, blpAB, are not intact in strain D39 and most other strains. By contrast, all sequenced pneumococcal strains contain intact comAB genes, encoding the transport system for CSP. Consistent with the idea that comAB mediate BlpC export, we finally show that high-level expression of the blp-genes requires comAB. Together, our results demonstrate that regulation of pneumocin expression is intertwined with competence, explaining why certain antibiotics induce blp-expression. Antibiotic-induced pneumocin expression might therefore have unpredictable consequences on pneumococcal colonization dynamics by activating genes that mediate intra-specific interference competition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

