Neisseria meningitidis Translation Elongation Factor P and Its Active-Site Arginine Residue Are Essential for Cell Viability
- PMID: 26840407
- PMCID: PMC4739656
- DOI: 10.1371/journal.pone.0147907
Neisseria meningitidis Translation Elongation Factor P and Its Active-Site Arginine Residue Are Essential for Cell Viability
Abstract
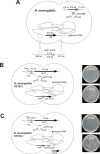
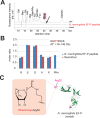
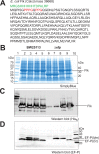
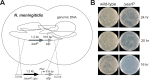
Translation elongation factor P (EF-P), a ubiquitous protein over the entire range of bacterial species, rescues ribosomal stalling at consecutive prolines in proteins. In Escherichia coli and Salmonella enterica, the post-translational β-lysyl modification of Lys34 of EF-P is important for the EF-P activity. The β-lysyl EF-P modification pathway is conserved among only 26-28% of bacteria. Recently, it was found that the Shewanella oneidensis and Pseudomonas aeruginosa EF-P proteins, containing an Arg residue at position 32, are modified with rhamnose, which is a novel post-translational modification. In these bacteria, EF-P and its Arg modification are both dispensable for cell viability, similar to the E. coli and S. enterica EF-P proteins and their Lys34 modification. However, in the present study, we found that EF-P and Arg32 are essential for the viability of the human pathogen, Neisseria meningitidis. We therefore analyzed the modification of Arg32 in the N. meningitidis EF-P protein, and identified the same rhamnosyl modification as in the S. oneidensis and P. aeruginosa EF-P proteins. N. meningitidis also has the orthologue of the rhamnosyl modification enzyme (EarP) from S. oneidensis and P. aeruginosa. Therefore, EarP should be a promising target for antibacterial drug development specifically against N. meningitidis. The pair of genes encoding N. meningitidis EF-P and EarP suppressed the slow-growth phenotype of the EF-P-deficient mutant of E. coli, indicating that the activity of N. meningitidis rhamnosyl-EF-P for rescuing the stalled ribosomes at proline stretches is similar to that of E. coli β-lysyl-EF-P. The possible reasons for the unique requirement of rhamnosyl-EF-P for N. meningitidis cells are that more proline stretch-containing proteins are essential and/or the basal ribosomal activity to synthesize proline stretch-containing proteins in the absence of EF-P is lower in this bacterium than in others.
Conflict of interest statement
Figures




Similar articles
-
Cyclic Rhamnosylated Elongation Factor P Establishes Antibiotic Resistance in Pseudomonas aeruginosa.mBio. 2015 Jun 9;6(3):e00823. doi: 10.1128/mBio.00823-15. mBio. 2015. PMID: 26060278 Free PMC article.
-
Arginine-rhamnosylation as new strategy to activate translation elongation factor P.Nat Chem Biol. 2015 Apr;11(4):266-70. doi: 10.1038/nchembio.1751. Epub 2015 Feb 16. Nat Chem Biol. 2015. PMID: 25686373 Free PMC article.
-
Complex Structure of Pseudomonas aeruginosa Arginine Rhamnosyltransferase EarP with Its Acceptor Elongation Factor P.J Bacteriol. 2019 Jun 10;201(13):e00128-19. doi: 10.1128/JB.00128-19. Print 2019 Jul 1. J Bacteriol. 2019. PMID: 31010899 Free PMC article.
-
Stall no more at polyproline stretches with the translation elongation factors EF-P and IF-5A.Mol Microbiol. 2016 Jan;99(2):219-35. doi: 10.1111/mmi.13233. Epub 2015 Nov 5. Mol Microbiol. 2016. PMID: 26416626 Review.
-
Bacteria-Catalyzed Arginine Glycosylation in Pathogens and Host.Front Cell Infect Microbiol. 2020 Apr 28;10:185. doi: 10.3389/fcimb.2020.00185. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32411621 Free PMC article. Review.
Cited by
-
A β-hairpin epitope as novel structural requirement for protein arginine rhamnosylation.Chem Sci. 2020 Dec 7;12(4):1560-1567. doi: 10.1039/d0sc05823h. Chem Sci. 2020. PMID: 34163919 Free PMC article.
-
Elongation factor P is required to maintain proteome homeostasis at high growth rate.Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):11072-11077. doi: 10.1073/pnas.1812025115. Epub 2018 Oct 8. Proc Natl Acad Sci U S A. 2018. PMID: 30297417 Free PMC article.
-
YfmR is a translation factor that prevents ribosome stalling and cell death in the absence of EF-P.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2314437121. doi: 10.1073/pnas.2314437121. Epub 2024 Feb 13. Proc Natl Acad Sci U S A. 2024. PMID: 38349882 Free PMC article.
-
Synthesis of rhamnosylated arginine glycopeptides and determination of the glycosidic linkage in bacterial elongation factor P.Chem Sci. 2017 Mar 1;8(3):2296-2302. doi: 10.1039/c6sc03847f. Epub 2016 Dec 12. Chem Sci. 2017. PMID: 28451332 Free PMC article.
-
Resolving the α-glycosidic linkage of arginine-rhamnosylated translation elongation factor P triggers generation of the first ArgRha specific antibody.Chem Sci. 2016 Dec 1;7(12):6995-7001. doi: 10.1039/c6sc02889f. Epub 2016 Jul 21. Chem Sci. 2016. PMID: 28451135 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

