Rapid Expansion of Human Epithelial Stem Cells Suitable for Airway Tissue Engineering
- PMID: 26840431
- PMCID: PMC5003214
- DOI: 10.1164/rccm.201507-1414OC
Rapid Expansion of Human Epithelial Stem Cells Suitable for Airway Tissue Engineering
Abstract
Rationale: Stem cell-based tracheal replacement represents an emerging therapeutic option for patients with otherwise untreatable airway diseases including long-segment congenital tracheal stenosis and upper airway tumors. Clinical experience demonstrates that restoration of mucociliary clearance in the lungs after transplantation of tissue-engineered grafts is critical, with preclinical studies showing that seeding scaffolds with autologous mucosa improves regeneration. High epithelial cell-seeding densities are required in regenerative medicine, and existing techniques are inadequate to achieve coverage of clinically suitable grafts.
Objectives: To define a scalable cell culture system to deliver airway epithelium to clinical grafts.
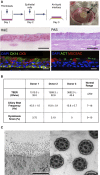
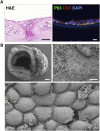
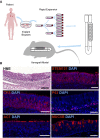
Methods: Human respiratory epithelial cells derived from endobronchial biopsies were cultured using a combination of mitotically inactivated fibroblasts and Rho-associated protein kinase (ROCK) inhibition using Y-27632 (3T3+Y). Cells were analyzed by immunofluorescence, quantitative polymerase chain reaction, and flow cytometry to assess airway stem cell marker expression. Karyotyping and multiplex ligation-dependent probe amplification were performed to assess cell safety. Differentiation capacity was tested in three-dimensional tracheospheres, organotypic cultures, air-liquid interface cultures, and an in vivo tracheal xenograft model. Ciliary function was assessed in air-liquid interface cultures.
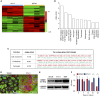
Measurements and main results: 3T3-J2 feeder cells and ROCK inhibition allowed rapid expansion of airway basal cells. These cells were capable of multipotent differentiation in vitro, generating both ciliated and goblet cell lineages. Cilia were functional with normal beat frequency and pattern. Cultured cells repopulated tracheal scaffolds in a heterotopic transplantation xenograft model.
Conclusions: Our method generates large numbers of functional airway basal epithelial cells with the efficiency demanded by clinical transplantation, suggesting its suitability for use in tracheal reconstruction.
Keywords: adult stem cells; epithelium; respiratory mucosa; tissue engineering; trachea.
Figures




Comment in
-
Airway Basal Cell Expansion Takes Cues from Keratinocytes.Am J Respir Crit Care Med. 2016 Jul 15;194(2):127-8. doi: 10.1164/rccm.201602-0366ED. Am J Respir Crit Care Med. 2016. PMID: 27420354 No abstract available.
References
-
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–171. - PubMed
-
- Ren X, Ott HC. On the road to bioartificial organs. Pflugers Arch. 2014;466:1847–1857. - PubMed
-
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. - PubMed
-
- Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D Leuven Tracheal Transplant Group. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

