Nogo-B receptor promotes the chemoresistance of human hepatocellular carcinoma via the ubiquitination of p53 protein
- PMID: 26840457
- PMCID: PMC4891009
- DOI: 10.18632/oncotarget.7091
Nogo-B receptor promotes the chemoresistance of human hepatocellular carcinoma via the ubiquitination of p53 protein
Abstract
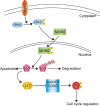
Nogo-B receptor (NgBR), a type I single transmembrane domain receptor is the specific receptor for Nogo-B. Our previous work demonstrated that NgBR is highly expressed in breast cancer cells, where it promotes epithelial mesenchymal transition (EMT), an important step in metastasis. Here, we show that both in vitro and in vivo increased expression of NgBR contributes to the increased chemoresistance of Bel7402/5FU cells, a stable 5-FU (5-Fluorouracil) resistant cell line related Bel7402 cells. NgBR knockdown abrogates S-phase arrest in Bel7402/5FU cells, which correlates with a reduction in G1/S phase checkpoint proteins p53 and p21. In addition, NgBR suppresses p53 protein levels through activation of the PI3K/Akt/MDM2 pathway, which promotes p53 degradation via the ubiquitin proteasome pathway and thus increases the resistance of human hepatocellular cancer cells to 5-FU. Furthermore, we found that NgBR expression is associated with a poor prognosis of human hepatocellular carcinoma (HCC) patients. These results suggest that targeting NgBR in combination with chemotherapeutic drugs, such as 5-FU, could improve the efficacy of current anticancer treatments.
Keywords: HCC; NgBR; chemoresistance.
Conflict of interest statement
The authors declare no conflicts of interest and no financial disclosures.
Figures






Similar articles
-
The Nogo-B receptor promotes human hepatocellular carcinoma cell growth via the Akt signal pathway.J Cell Biochem. 2018 Sep;119(9):7738-7746. doi: 10.1002/jcb.27125. Epub 2018 Jun 15. J Cell Biochem. 2018. PMID: 29904947 Free PMC article.
-
FAM9C plays an anti-apoptotic role through activation of the PI3K/Akt pathway in human hepatocellular carcinoma.Oncol Rep. 2013 Sep;30(3):1275-84. doi: 10.3892/or.2013.2592. Epub 2013 Jul 5. Oncol Rep. 2013. PMID: 23836295
-
NKD1 correlates with a poor prognosis and inhibits cell proliferation by inducing p53 expression in hepatocellular carcinoma.Tumour Biol. 2016 Oct;37(10):14059-14067. doi: 10.1007/s13277-016-5173-0. Epub 2016 Aug 9. Tumour Biol. 2016. PMID: 27507614
-
MDM2-p53 pathway in hepatocellular carcinoma.Cancer Res. 2014 Dec 15;74(24):7161-7. doi: 10.1158/0008-5472.CAN-14-1446. Epub 2014 Dec 4. Cancer Res. 2014. PMID: 25477334 Free PMC article. Review.
-
Neurite Outgrowth Inhibitor B Receptor: A Versatile Receptor with Multiple Functions and Actions.DNA Cell Biol. 2017 Dec;36(12):1142-1150. doi: 10.1089/dna.2017.3813. Epub 2017 Oct 23. DNA Cell Biol. 2017. PMID: 29058484 Review.
Cited by
-
Salivary NUS1 and RCN1 Levels as Biomarkers for Oral Squamous Cell Carcinoma Diagnosis.In Vivo. 2020 Sep-Oct;34(5):2353-2361. doi: 10.21873/invivo.12048. In Vivo. 2020. PMID: 32871760 Free PMC article.
-
Nogo-B promotes tumor angiogenesis and provides a potential therapeutic target in hepatocellular carcinoma.Mol Oncol. 2018 Dec;12(12):2042-2054. doi: 10.1002/1878-0261.12358. Epub 2018 Oct 26. Mol Oncol. 2018. PMID: 30019429 Free PMC article.
-
Nogo-B receptor is required for stabilizing TGF-β type I receptor and promotes the TGF-β1-induced epithelial-to-mesenchymal transition of non-small cell lung cancer.J Cancer. 2021 Jan 1;12(3):717-725. doi: 10.7150/jca.50483. eCollection 2021. J Cancer. 2021. PMID: 33403029 Free PMC article.
-
Epigenetically Down-Regulated Acetyltransferase PCAF Increases the Resistance of Colorectal Cancer to 5-Fluorouracil.Neoplasia. 2019 Jun;21(6):557-570. doi: 10.1016/j.neo.2019.03.011. Epub 2019 Apr 28. Neoplasia. 2019. PMID: 31042625 Free PMC article.
-
The co-treatment of metformin with flavone synergistically induces apoptosis through inhibition of PI3K/AKT pathway in breast cancer cells.Oncol Lett. 2018 Apr;15(4):5952-5958. doi: 10.3892/ol.2018.7999. Epub 2018 Feb 8. Oncol Lett. 2018. PMID: 29552226 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
-
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

