Simvastatin Efficiently Lowers Small LDL-IgG Immune Complex Levels: A Therapeutic Quality beyond the Lipid-Lowering Effect
- PMID: 26840480
- PMCID: PMC4739583
- DOI: 10.1371/journal.pone.0148210
Simvastatin Efficiently Lowers Small LDL-IgG Immune Complex Levels: A Therapeutic Quality beyond the Lipid-Lowering Effect
Abstract
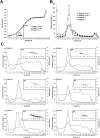
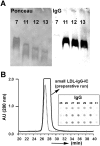
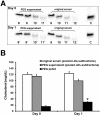
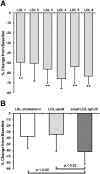
We investigated a polyethylene glycol non-precipitable low-density lipoprotein (LDL) subfraction targeted by IgG and the influence of statin therapy on plasma levels of these small LDL-IgG-immune complexes (LDL-IgG-IC). LDL-subfractions were isolated from 6 atherosclerotic subjects and 3 healthy individuals utilizing iodixanol density gradient ultracentrifugation. Cholesterol, apoB and malondialdehyde (MDA) levels were determined in each fraction by enzymatic testing, dissociation-enhanced lanthanide fluorescence immunoassay and high-performance liquid chromatography, respectively. The levels of LDL-IgG-IC were quantified densitometrically following lipid electrophoresis, particle size distribution was assessed with dynamic light scattering and size exclusion chromatography. The influence of simvastatin (40 mg/day for three months) on small LDL-IgG-IC levels and their distribution among LDL-subfractions (salt gradient separation) were investigated in 11 patients with confirmed coronary artery disease (CAD). We demonstrate that the investigated LDL-IgG-IC are small particles present in atherosclerotic patients and healthy subjects. In vitro assembly of LDL-IgG-IC resulted in particle density shifts indicating a composition of one single molecule of IgG per LDL particle. Normalization on cholesterol levels revealed MDA values twice as high for LDL-subfractions rich in small LDL-IgG-IC if compared to dominant LDL-subfractions. Reactivity of affinity purified small LDL-IgG-IC to monoclonal antibody OB/04 indicates a high degree of modified apoB and oxidative modification. Simvastatin therapy studied in the CAD patients significantly lowered LDL levels and to an even higher extent, small LDL-IgG-IC levels without affecting their distribution. In conclusion simvastatin lowers levels of small LDL-IgG-IC more effectively than LDL-cholesterol and LDL-apoB levels in atherosclerotic patients. This antiatherogenic effect may additionally contribute to the known beneficial effects of this drug in the treatment of atherosclerosis.
Conflict of interest statement
Figures

Similar articles
-
Effects of ezetimibe/simvastatin on lipoprotein subfractions in patients with primary hypercholesterolemia: an exploratory analysis of archived samples using two commercially available techniques.Clin Ther. 2007 Nov;29(11):2419-32. doi: 10.1016/j.clinthera.2007.10.004. Clin Ther. 2007. PMID: 18158082 Clinical Trial.
-
Pitavastatin: novel effects on lipid parameters.Atheroscler Suppl. 2011 Nov;12(3):277-84. doi: 10.1016/S1567-5688(11)70887-X. Atheroscler Suppl. 2011. PMID: 22152282 Review.
-
Relationship between plasma phospholipase A2 concentrations and lipoprotein subfractions in patients with stable coronary artery disease.Clin Chim Acta. 2015 Jun 15;446:195-200. doi: 10.1016/j.cca.2015.04.032. Epub 2015 Apr 28. Clin Chim Acta. 2015. PMID: 25934512
-
Effect of simvastatin in familial hypercholesterolemia on the affinity of electronegative low-density lipoprotein subfractions to the low-density lipoprotein receptor.Am J Cardiol. 2004 Feb 15;93(4):414-20. doi: 10.1016/j.amjcard.2003.10.034. Am J Cardiol. 2004. PMID: 14969613
-
2017 Taiwan lipid guidelines for high risk patients.J Formos Med Assoc. 2017 Apr;116(4):217-248. doi: 10.1016/j.jfma.2016.11.013. Epub 2017 Feb 24. J Formos Med Assoc. 2017. PMID: 28242176 Review.
Cited by
-
Circulating Anti-Coatomer Protein Complex Subunit Epsilon (COPE) Autoantibodies as a Potential Biomarker for Cardiovascular and Cerebrovascular Events in Patients with Obstructive Sleep Apnea.J Clin Sleep Med. 2017 Mar 15;13(3):393-400. doi: 10.5664/jcsm.6488. J Clin Sleep Med. 2017. PMID: 27923433 Free PMC article.
-
Anti-atherosclerotic effects between a combined treatment with simvastatin plus hirudin and single simvastatin therapy in patients with early type 2 diabetes mellitus.Ann Transl Med. 2019 Jul;7(14):302. doi: 10.21037/atm.2019.05.69. Ann Transl Med. 2019. PMID: 31475172 Free PMC article.
-
Ethyl pyruvate inhibits oxidation of LDL in vitro and attenuates oxLDL toxicity in EA.hy926 cells.PLoS One. 2018 Jan 25;13(1):e0191477. doi: 10.1371/journal.pone.0191477. eCollection 2018. PLoS One. 2018. PMID: 29370236 Free PMC article.
-
Utility of atherosclerosis-associated serum antibodies against colony-stimulating factor 2 in predicting the onset of acute ischemic stroke and prognosis of colorectal cancer.Front Cardiovasc Med. 2023 Feb 10;10:1042272. doi: 10.3389/fcvm.2023.1042272. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36844744 Free PMC article.
-
Immune Complexes and the Risk of CVD in Type 1 Diabetes.Diabetes. 2019 Sep;68(9):1853-1860. doi: 10.2337/db19-0358. Epub 2019 Jun 19. Diabetes. 2019. PMID: 31217176 Free PMC article. Clinical Trial.
References
-
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–24. - PubMed
-
- Orekhov AN, Bobryshev YV, Sobenin IA, Melnichenko AA, Chistiakov DA. Modified low density lipoprotein and lipoprotein-containing circulating immune complexes as diagnostic and prognostic biomarkers of atherosclerosis and type 1 diabetes macrovascular disease. International journal of molecular sciences. 2014;15(7):12807–41. 10.3390/ijms150712807 - DOI - PMC - PubMed
-
- Matsuura E, Kobayashi K, Lopez LR. Atherosclerosis in autoimmune diseases. Curr Rheumatol Rep. 2009;11(1):61–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

