A thalamic input to the nucleus accumbens mediates opiate dependence
- PMID: 26840481
- PMCID: PMC4814115
- DOI: 10.1038/nature16954
A thalamic input to the nucleus accumbens mediates opiate dependence
Abstract
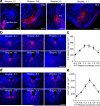
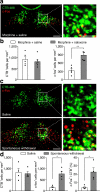
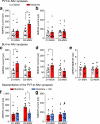
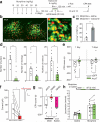
Chronic opiate use induces opiate dependence, which is characterized by extremely unpleasant physical and emotional feelings after drug use is terminated. Both the rewarding effects of a drug and the desire to avoid withdrawal symptoms motivate continued drug use, and the nucleus accumbens is important for orchestrating both processes. While multiple inputs to the nucleus accumbens regulate reward, little is known about the nucleus accumbens circuitry underlying withdrawal. Here we identify the paraventricular nucleus of the thalamus as a prominent input to the nucleus accumbens mediating the expression of opiate-withdrawal-induced physical signs and aversive memory. Activity in the paraventricular nucleus of the thalamus to nucleus accumbens pathway is necessary and sufficient to mediate behavioural aversion. Selectively silencing this pathway abolishes aversive symptoms in two different mouse models of opiate withdrawal. Chronic morphine exposure selectively potentiates excitatory transmission between the paraventricular nucleus of the thalamus and D2-receptor-expressing medium spiny neurons via synaptic insertion of GluA2-lacking AMPA receptors. Notably, in vivo optogenetic depotentiation restores normal transmission at these synapses and robustly suppresses morphine withdrawal symptoms. This links morphine-evoked pathway- and cell-type-specific plasticity in the paraventricular nucleus of the thalamus to nucleus accumbens circuit to opiate dependence, and suggests that reprogramming this circuit holds promise for treating opiate addiction.
Figures









Comment in
-
Addiction: Road to aversion.Nat Rev Neurosci. 2016 Apr;17(4):200. doi: 10.1038/nrn.2016.25. Epub 2016 Feb 25. Nat Rev Neurosci. 2016. PMID: 26911434 No abstract available.
References
-
- Koob GF, LeMoal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi:DOI 10.1126/science.278.5335.52. - PubMed
-
- Vargas-Perez H, Ting AKRA, Heinmiller A, Sturgess JE, van der Kooy D. A test of the opponent-process theory of motivation using lesions that selectively block morphine reward. Eur J Neurosci. 2007;25:3713–3718. doi:10.1111/j.1460-9568.2007.05599.x. - PubMed
-
- Wikler A. A theory of opioid dependence. NIDA Res Monogr. 1980;30:174–178. - PubMed
-
- Harris GC, Astonjones G. Involvement of D2 Dopamine-Receptors in the Nucleus-Accumbens in the Opiate Withdrawal Syndrome. Nature. 1994;371:155–157. doi:Doi 10.1038/371155a0. - PubMed
-
- Koob GF, Wall TL, Bloom FE. Nucleus Accumbens as a Substrate for the Aversive Stimulus Effects of Opiate Withdrawal. Psychopharmacology. 1989;98:530–534. doi:Doi 10.1007/Bf00441954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

