Re-engineering the zinc fingers of PRDM9 reverses hybrid sterility in mice
- PMID: 26840484
- PMCID: PMC4756437
- DOI: 10.1038/nature16931
Re-engineering the zinc fingers of PRDM9 reverses hybrid sterility in mice
Abstract
The DNA-binding protein PRDM9 directs positioning of the double-strand breaks (DSBs) that initiate meiotic recombination in mice and humans. Prdm9 is the only mammalian speciation gene yet identified and is responsible for sterility phenotypes in male hybrids of certain mouse subspecies. To investigate PRDM9 binding and its role in fertility and meiotic recombination, we humanized the DNA-binding domain of PRDM9 in C57BL/6 mice. This change repositions DSB hotspots and completely restores fertility in male hybrids. Here we show that alteration of one Prdm9 allele impacts the behaviour of DSBs controlled by the other allele at chromosome-wide scales. These effects correlate strongly with the degree to which each PRDM9 variant binds both homologues at the DSB sites it controls. Furthermore, higher genome-wide levels of such 'symmetric' PRDM9 binding associate with increasing fertility measures, and comparisons of individual hotspots suggest binding symmetry plays a downstream role in the recombination process. These findings reveal that subspecies-specific degradation of PRDM9 binding sites by meiotic drive, which steadily increases asymmetric PRDM9 binding, has impacts beyond simply changing hotspot positions, and strongly support a direct involvement in hybrid infertility. Because such meiotic drive occurs across mammals, PRDM9 may play a wider, yet transient, role in the early stages of speciation.
Figures




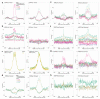
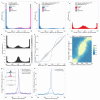


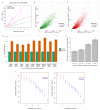
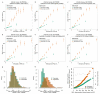



Comment in
-
Genetics: Asymmetric breaks in DNA cause sterility.Nature. 2016 Feb 11;530(7589):167-8. doi: 10.1038/nature16870. Epub 2016 Feb 3. Nature. 2016. PMID: 26840487 No abstract available.
-
Evolution: On the Origin of Symmetry, Synapsis, and Species.Curr Biol. 2016 Apr 25;26(8):R325-8. doi: 10.1016/j.cub.2016.03.014. Curr Biol. 2016. PMID: 27115689
References
-
- Presgraves DC. The molecular evolutionary basis of species formation. Nat Rev Genet. 2010;11:175–180. 10.1038/nrg2718. - PubMed
-
- Mihola O, Trachtulec Z, Vlcek C, Schimenti JC, Forejt J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science. 2009;323:373–375. 10.1126/science.1163601. - PubMed
-
- Gregorova S, Forejt J. PWD/Ph and PWK/Ph inbred mouse strains of Mus m. musculus subspecies--a valuable resource of phenotypic variations and genomic polymorphisms. Folia biologica. 2000;46:31–41. - PubMed
Additional references for methods
-
- Peters AH, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 1997;5:66–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

