Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans
- PMID: 26840628
- PMCID: PMC4887698
- DOI: 10.1113/JP271836
Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans
Abstract
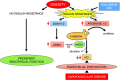
Key points: The presence of insulin resistance (IR) is determinant for endothelial dysfunction associated with obesity. Although recent studies have implicated the involvement of mitochondrial superoxide and inflammation in the defective nitric oxide (NO)-mediated responses and subsequent endothelial dysfunction in IR, other mechanisms could compromise this pathway. In the present study, we assessed the role of asymmetric dimethylarginine (ADMA) and arginase with respect to IR-induced impairment of endothelium-dependent vasodilatation in human morbid obesity and in a non-obese rat model of IR. We show that both increased ADMA and up-regulated arginase are determinant factors in the alteration of the l-arginine/NO pathway associated with IR in both models and also that acute treatment of arteries with arginase inhibitor or with l-arginine significantly alleviate endothelial dysfunction. These results help to expand our knowledge regarding the mechanisms of endothelial dysfunction that are related to obesity and IR and establish potential therapeutic targets for intervention.
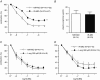
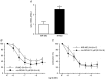
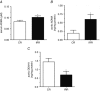
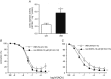
Abstract: Insulin resistance (IR) is determinant for endothelial dysfunction in human obesity. Although we have previously reported the involvement of mitochondrial superoxide and inflammation, other mechanisms could compromise NO-mediated responses in IR. We evaluated the role of the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA) and arginase with respect to IR-induced impairment of l-arginine/NO-mediated vasodilatation in human morbid obesity and in a non-obese rat model of IR. Bradykinin-induced vasodilatation was evaluated in microarteries derived from insulin-resistant morbidly obese (IR-MO) and non-insulin-resistant MO (NIR-MO) subjects. Defective endothelial vasodilatation in IR-MO was improved by l-arginine supplementation. Increased levels of ADMA were detected in serum and adipose tissue from IR-MO. Serum ADMA positively correlated with IR score and negatively with pD2 for bradykinin. Gene expression determination by RT-PCR revealed not only the decreased expression of ADMA degrading enzyme dimethylarginine dimethylaminohydrolase (DDAH)1/2 in IR-MO microarteries, but also increased expression of arginase-2. Arginase inhibition improved endothelial vasodilatation in IR-MO. Analysis of endothelial vasodilatation in a non-obese IR model (fructose-fed rat) confirmed an elevation of circulating and aortic ADMA concentrations, as well as reduced DDAH aortic content and increased aortic arginase activity in IR. Improvement of endothelial vasodilatation in IR rats by l-arginine supplementation and arginase inhibition provided functional corroboration. These results demonstrate that increased ADMA and up-regulated arginase contribute to endothelial dysfunction as determined by the presence of IR in human obesity, most probably by compromising arginine availability. The results provide novel insights regarding the mechanisms of endothelial dysfunction related to obesity and IR and establish potential therapeutic targets for intervention.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


Comment in
-
Strict glucose control and artificial regulation of the NO-ADMA-DDAH system in order to prevent endothelial dysfunction.J Physiol. 2016 Jun 1;594(11):2775-6. doi: 10.1113/JP272183. J Physiol. 2016. PMID: 27246541 Free PMC article. No abstract available.
References
-
- Anderssohn M, Schwedhelm E, Luneburg N, Vasan RS & Boger RH (2010). Asymmetric dimethylarginine as a mediator of vascular dysfunction and a marker of cardiovascular disease and mortality: an intriguing interaction with diabetes mellitus. Diab Vasc Dis Res 7, 105–118. - PubMed
-
- Angulo J, Sanchez‐Ferrer CF, Peiro C, Marin J & Rodriguez‐Manas L (1996). Impairment of endothelium‐dependent relaxation by increasing percentages of glycosylated human hemoglobin. Possible mechanisms involved. Hypertension 28, 583–592. - PubMed
-
- Asagami T, Abbasi F, Stuelinger M, Lamendola C, McLaughlin T, Cooke JP, Reaven GM & Tsao PS (2002). Metformin treatment lowers asymmetric dimethylarginine concentrations in patients with type 2 diabetes. Metabolism 51, 843–846. - PubMed
-
- Ascaso JF, Romero P, Real JT, Priego A, Valdecabres C & Carmena R (2001). [Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non‐diabetic population]. Med Clin (Barc) 117, 530–533. - PubMed
-
- Boger RH (2004). Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the ‘L‐arginine paradox’ and acts as a novel cardiovascular risk factor. J Nutr 134, 2842S‐2847S; discussion 2853S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

