Ca2+ Effects on ATP Production and Consumption Have Regulatory Roles on Oscillatory Islet Activity
- PMID: 26840737
- PMCID: PMC4744176
- DOI: 10.1016/j.bpj.2015.11.3526
Ca2+ Effects on ATP Production and Consumption Have Regulatory Roles on Oscillatory Islet Activity
Abstract
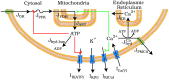
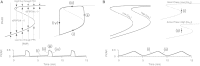
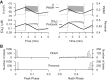
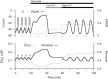
Pancreatic islets respond to elevated blood glucose by secreting pulses of insulin that parallel oscillations in β-cell metabolism, intracellular Ca(2+) concentration, and bursting electrical activity. The mechanisms that maintain an oscillatory response are not fully understood, yet several models have been proposed. Only some can account for experiments supporting that metabolism is intrinsically oscillatory in β-cells. The dual oscillator model (DOM) implicates glycolysis as the source of oscillatory metabolism. In the companion article, we use recently developed biosensors to confirm that glycolysis is oscillatory and further elucidate the coordination of metabolic and electrical signals in the insulin secretory pathway. In this report, we modify the DOM by incorporating an established link between metabolism and intracellular Ca(2+) to reconcile model predictions with experimental observations from the companion article. With modification, we maintain the distinguishing feature of the DOM, oscillatory glycolysis, but introduce the ability of Ca(2+) influx to reshape glycolytic oscillations by promoting glycolytic efflux. We use the modified model to explain measurements from the companion article and from previously published experiments with islets.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Phase Analysis of Metabolic Oscillations and Membrane Potential in Pancreatic Islet β-Cells.Biophys J. 2016 Feb 2;110(3):691-699. doi: 10.1016/j.bpj.2015.12.029. Biophys J. 2016. PMID: 26840733 Free PMC article.
-
Transitions between bursting modes in the integrated oscillator model for pancreatic β-cells.J Theor Biol. 2018 Oct 7;454:310-319. doi: 10.1016/j.jtbi.2018.06.017. Epub 2018 Jun 20. J Theor Biol. 2018. PMID: 29935201
-
Are metabolic oscillations responsible for normal oscillatory insulin secretion?Diabetes. 1997 Sep;46(9):1375-80. doi: 10.2337/diab.46.9.1375. Diabetes. 1997. PMID: 9287034
-
Electrophysiology of islet cells.Adv Exp Med Biol. 2010;654:115-63. doi: 10.1007/978-90-481-3271-3_7. Adv Exp Med Biol. 2010. PMID: 20217497 Review.
-
Bursting and calcium oscillations in pancreatic beta-cells: specific pacemakers for specific mechanisms.Am J Physiol Endocrinol Metab. 2010 Oct;299(4):E517-32. doi: 10.1152/ajpendo.00177.2010. Epub 2010 Jul 13. Am J Physiol Endocrinol Metab. 2010. PMID: 20628025 Free PMC article. Review.
Cited by
-
CDK2 limits the highly energetic secretory program of mature β cells by restricting PEP cycle-dependent KATP channel closure.Cell Rep. 2021 Jan 26;34(4):108690. doi: 10.1016/j.celrep.2021.108690. Cell Rep. 2021. PMID: 33503433 Free PMC article.
-
Symbiosis of Electrical and Metabolic Oscillations in Pancreatic β-Cells.Front Physiol. 2021 Dec 3;12:781581. doi: 10.3389/fphys.2021.781581. eCollection 2021. Front Physiol. 2021. PMID: 34925070 Free PMC article. Review.
-
Phase Analysis of Metabolic Oscillations and Membrane Potential in Pancreatic Islet β-Cells.Biophys J. 2016 Feb 2;110(3):691-699. doi: 10.1016/j.bpj.2015.12.029. Biophys J. 2016. PMID: 26840733 Free PMC article.
-
Spatial distribution of heterogeneity as a modulator of collective dynamics in pancreatic beta-cell networks and beyond.Front Netw Physiol. 2023 Mar 24;3:1170930. doi: 10.3389/fnetp.2023.1170930. Front Netw Physiol. 2023. PMID: 36987428 Free PMC article.
-
Cholesterol Concentration in Cell Membranes and its Impact on Receptor-Ligand Interaction: A Computational Study of ATP-Sensitive Potassium Channels and ATP Binding.J Membr Biol. 2025 Jun;258(3):225-236. doi: 10.1007/s00232-025-00345-4. Epub 2025 Mar 26. J Membr Biol. 2025. PMID: 40137942 Free PMC article.
References
-
- Weigle D.S. Pulsatile secretion of fuel-regulatory hormones. Diabetes. 1987;36:764–775. - PubMed
-
- Bertram R., Sherman A., Satin L.S. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2007;293:E890–E900. - PubMed
-
- Meier J.J., Veldhuis J.D., Butler P.C. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. - PubMed
-
- Bratusch-Marrain P.R., Komjati M., Waldhäusl W.K. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986;35:922–926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

