Triggering Dectin-1-Pathway Alone Is Not Sufficient to Induce Cytokine Production by Murine Macrophages
- PMID: 26840954
- PMCID: PMC4739705
- DOI: 10.1371/journal.pone.0148464
Triggering Dectin-1-Pathway Alone Is Not Sufficient to Induce Cytokine Production by Murine Macrophages
Abstract
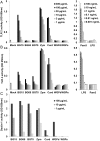
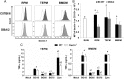
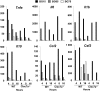
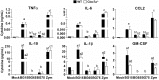
β-glucans (BG) are abundant polysaccharides of the Saccharomyces cerevisiae cell wall (Sc CW), an industry byproduct. They have immuno-stimulatory properties upon engagement of dectin-1 (Clec7a), their main receptor on particular immune cells, and they actually become of great interest because of their preventive or therapeutic potentials. Zymosan, a crude extract of Sc CW was studied as a prototypic BG, despite its miscellaneous PAMPs content. Here, we examined the response of murine wild type or Clec7a-/- bone marrow-derived macrophages (BMDM) to products with increasing BG content (15, 65 or 75%) and compared their effects with those of other dectin-1 ligands. The enrichment process removed TLR ligands while preserving dectin-1 activity. The most enriched extracts have very low NFκB activity and triggered low amounts of cytokine production in contrast with crude products like zymosan and BG15. Furthermore, MyD88-/- BMDM did not produce TNFα in response to crude Sc CW extracts, whereas their response to BG-enriched extracts was unaffected, suggesting that BG alone are not able to initiate cytokine secretion. Although Sc CW-derived BG stimulated the late and strong expression of Csf2 in a dectin-1-dependent manner, they remain poor inducers of chemokine and cytokine production in murine macrophages.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

