Antenatal Corticosteroids for Reducing Adverse Maternal and Child Outcomes in Special Populations of Women at Risk of Imminent Preterm Birth: A Systematic Review and Meta-Analysis
- PMID: 26841022
- PMCID: PMC4740425
- DOI: 10.1371/journal.pone.0147604
Antenatal Corticosteroids for Reducing Adverse Maternal and Child Outcomes in Special Populations of Women at Risk of Imminent Preterm Birth: A Systematic Review and Meta-Analysis
Abstract
Background: This study synthesizes available evidence on antenatal corticosteroids (ACS) use among special subgroups of women at risk of imminent preterm birth, including those (1) with pregestational and gestational diabetes mellitus, (2) undergoing elective caesarean section (CS) in late preterm (34 to<37 weeks), (3) with chorioamnionitis, and (4) with growth-restricted fetuses.
Methods: A systematic search of MEDLINE, EMBASE, CINAHL, Cochrane Library, POPLINE, and World Health Organization Regional Databases was conducted for all comparative studies. Two reviewers independently determined study eligibility, extracted data, and assessed study quality. Pooled mean differences and odds ratios with 95% confidence intervals were estimated from available data, based on fixed- and random-effects models, as appropriate.
Results: No eligible studies were identified for ACS use in diabetic pregnant women or those undergoing elective CS at late preterm. Nine studies each on ACS use in women with chorioamnionitis and in women with fetal growth restriction met inclusion criteria; eight studies were separately included in the meta-analyses for the two subpopulations. For ACS administration in women with chorioamnionitis, pooled analyses showed reductions in neonatal mortality (OR: 0.49, 95% CI: 0.34-0.73), respiratory distress syndrome (OR: 0.58, 95% CI: 0.44-0.76), intraventricular haemorrhage (OR: 0.41, 95% CI: 0.24-0.69), and severe intraventricular haemorrhage (OR: 0.40, 95% CI: 0.24-0.69). Maternal and long-term newborn outcomes were not reported. Effects of ACS use were inconclusive for cases with fetal growth restriction.
Conclusion: Direct evidence on the effectiveness and safety of ACS is lacking for diabetic pregnant women at risk of preterm birth and those undergoing elective late-preterm CS, though this does not necessarily recommend against their use in diabetic women. While evidence remains inconclusive for women with growth-restricted preterm neonates, ACS appears to benefit preterm neonates delivered by women with chorioamnionitis. High-quality studies on maternal and long-term child outcomes in more diverse settings are needed to establish the balance of potential harms versus benefits in using ACS for these understudied subgroups.
Conflict of interest statement
Figures
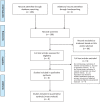



References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. 10.1016/s0140-6736(12)60820-4 . - DOI - PubMed
-
- Butler AS, Behrman RE. Preterm Birth: Causes, Consequences, and Prevention: Washington, DC: National Academies Press; 2007. - PubMed
-
- NIH. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413–8. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

