Innate Immunity and Inflammation in NAFLD/NASH
- PMID: 26841783
- PMCID: PMC4948286
- DOI: 10.1007/s10620-016-4049-x
Innate Immunity and Inflammation in NAFLD/NASH
Abstract
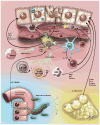
Inflammation and hepatocyte injury and death are the hallmarks of nonalcoholic steatohepatitis (NASH), the progressive form of nonalcoholic fatty liver disease (NAFLD), which is a currently burgeoning public health problem. Innate immune activation is a key factor in triggering and amplifying hepatic inflammation in NAFLD/NASH. Thus, identification of the underlying mechanisms by which immune cells in the liver recognize cell damage signals or the presence of pathogens or pathogen-derived factors that activate them is relevant from a therapeutic perspective. In this review, we present new insights into the factors promoting the inflammatory response in NASH including sterile cell death processes resulting from lipotoxicity in hepatocytes as well as into the altered gut-liver axis function, which involves translocation of bacterial products into portal circulation as a result of gut leakiness. We further delineate the key immune cell types involved and how they recognize both damage-associated molecular patterns or pathogen-associated molecular patterns through binding of surface-expressed pattern recognition receptors, which initiate signaling cascades leading to injury amplification. The relevance of modulating these inflammatory signaling pathways as potential novel therapeutic strategies for the treatment of NASH is summarized.
Keywords: Cell death; Inflammation; Innate immunity; Liver disease; NASH; Therapy.
Conflict of interest statement
Figures
References
-
- Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221–235. - PubMed
-
- Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. - PubMed
-
- Burt AD, Lackner C, Tiniakos DG. Diagnosis and Assessment of NAFLD: Definitions and Histopathological Classification. Semin Liver Dis. 2015;35:207–220. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

