Sorting Motifs in the Cytoplasmic Tail of the Immunomodulatory E3/49K Protein of Species D Adenoviruses Modulate Cell Surface Expression and Ectodomain Shedding
- PMID: 26841862
- PMCID: PMC4807268
- DOI: 10.1074/jbc.M115.684787
Sorting Motifs in the Cytoplasmic Tail of the Immunomodulatory E3/49K Protein of Species D Adenoviruses Modulate Cell Surface Expression and Ectodomain Shedding
Abstract
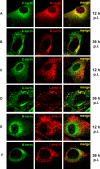
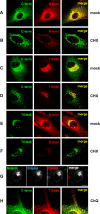
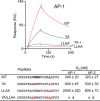
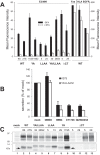
The E3 transcription unit of human species C adenoviruses (Ads) encodes immunomodulatory proteins that mediate direct protection of infected cells. Recently, we described a novel immunomodulatory function for E3/49K, an E3 protein uniquely expressed by species D Ads. E3/49K of Ad19a/Ad64, a serotype that causes epidemic keratokonjunctivitis, is synthesized as a highly glycosylated type I transmembrane protein that is subsequently cleaved, resulting in secretion of its large ectodomain (sec49K). sec49K binds to CD45 on leukocytes, impairing activation and functions of natural killer cells and T cells. E3/49K is localized in the Golgi/trans-Golgi network (TGN), in the early endosomes, and on the plasma membrane, yet the cellular compartment where E3/49K is cleaved and the protease involved remained elusive. Here we show that TGN-localized E3/49K comprises both newly synthesized and recycled molecules. Full-length E3/49K was not detected in late endosomes/lysosomes, but the C-terminal fragment accumulated in this compartment at late times of infection. Inhibitor studies showed that cleavage occurs in a post-TGN compartment and that lysosomotropic agents enhance secretion. Interestingly, the cytoplasmic tail of E3/49K contains two potential sorting motifs, YXXΦ (where Φ represents a bulky hydrophobic amino acid) and LL, that are important for binding the clathrin adaptor proteins AP-1 and AP-2in vitro Surprisingly, mutating the LL motif, either alone or together with YXXΦ, did not prevent proteolytic processing but increased cell surface expression and secretion. Upon brefeldin A treatment, cell surface expression was rapidly lost, even for mutants lacking all known endocytosis motifs. Together with immunofluorescence data, we propose a model for intracellular E3/49K transport whereby cleavage takes place on the cell surface by matrix metalloproteases.
Keywords: Adenovirus E3 proteins; CD45; E3/49K; Epidemic Keratoconjunctivitis; Immune evasion; adenovirus; intracellular trafficking; protein sorting; shedding; viral immunology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- King A. M. Q., Adams M. J., Carstens E. B., and Lefkowitz E. J. (eds) (2012) Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, pp. 213–228, Elsevier Academic Press, Amsterdam
-
- Wold W. S. M., and Horwitz M. S. (2007) in Fields Virology (Knipe D. M., Howley P. M., Griffin D. E., and Lamb R. A., eds) pp. 2395–2436, 5th Ed., Lippincott Williams & Wilkins, Philadelphia
-
- Fox J. P., Hall C. E., and Cooney M. K. (1977) The Seattle virus watch. VII. Observations of adenovirus infections. Am. J. Epidemiol. 105, 362–386 - PubMed
-
- Chodosh J., Miller D., Stroop W. G., and Pflugfelder S. C. (1995) Adenovirus epithelial keratitis. Cornea 14, 167–174 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

