Pharmacological removal of serum amyloid P component from intracerebral plaques and cerebrovascular Aβ amyloid deposits in vivo
- PMID: 26842068
- PMCID: PMC4772805
- DOI: 10.1098/rsob.150202
Pharmacological removal of serum amyloid P component from intracerebral plaques and cerebrovascular Aβ amyloid deposits in vivo
Abstract
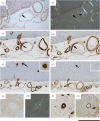
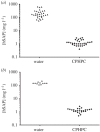
Human amyloid deposits always contain the normal plasma protein serum amyloid P component (SAP), owing to its avid but reversible binding to all amyloid fibrils, including the amyloid β (Aβ) fibrils in the cerebral parenchyma plaques and cerebrovascular amyloid deposits of Alzheimer's disease (AD) and cerebral amyloid angiopathy (CAA). SAP promotes amyloid fibril formation in vitro, contributes to persistence of amyloid in vivo and is also itself directly toxic to cerebral neurons. We therefore developed (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC), a drug that removes SAP from the blood, and thereby also from the cerebrospinal fluid (CSF), in patients with AD. Here we report that, after introduction of transgenic human SAP expression in the TASTPM double transgenic mouse model of AD, all the amyloid deposits contained human SAP. Depletion of circulating human SAP by CPHPC administration in these mice removed all detectable human SAP from both the intracerebral and cerebrovascular amyloid. The demonstration that removal of SAP from the blood and CSF also removes it from these amyloid deposits crucially validates the strategy of the forthcoming 'Depletion of serum amyloid P component in Alzheimer's disease (DESPIAD)' clinical trial of CPHPC. The results also strongly support clinical testing of CPHPC in patients with CAA.
Keywords: Alzheimer's disease; Aβ amyloid; CPHPC; cerebral amyloid angiopathy; serum amyloid P component.
© 2016 The Authors.
Figures



References
-
- Glenner GG, Wong CW. 1984. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122, 1131–1135. (doi:10.1016/0006-291X(84)91209-9) - DOI - PubMed
-
- De Strooper B, König G. 1999. Alzheimer's disease. A firm base for drug development. Nature 402, 471–472. (doi:10.1038/44973) - DOI - PubMed
-
- Brunkan AL, Goate AM. 2005. Presenilin function and γ-secretase activity. J. Neurochem. 93, 769–792. (doi:10.1111/j.1471-4159.2005.03099.x) - DOI - PubMed
-
- Bohm C, Chen F, Sevalle J, Qamar S, Dodd R, Li Y, Schmitt-Ulms G, Fraser PE, St George-Hyslop PH. 2015. Current and future implications of basic and translational research on amyloid-β peptide production and removal pathways. Mol. Cell. Neurosci. 66, 3–11. (doi:10.1016/j.mcn.2015.02.016) - DOI - PMC - PubMed
-
- Schneider LS, et al. 2014. Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. J. Intern. Med. 275, 251–283. (doi:10.1111/joim.12191) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
