Alcohol Decreases Organic Dust-Stimulated Airway Epithelial TNF-Alpha Through a Nitric Oxide and Protein Kinase-Mediated Inhibition of TACE
- PMID: 26842246
- PMCID: PMC5656047
- DOI: 10.1111/acer.12967
Alcohol Decreases Organic Dust-Stimulated Airway Epithelial TNF-Alpha Through a Nitric Oxide and Protein Kinase-Mediated Inhibition of TACE
Abstract
Background: Farm workers in rural areas consume more alcohol than those who reside in urban areas. Occupational exposures such as agricultural work can pose hazards on the respiratory system. It is established that hog barn dust induces inflammation in the airway, including the release of cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8. We have shown that alcohol alters airway epithelial innate defense through changes in both nitric oxide (NO) and cAMP-dependent protein kinase A (PKA). Simultaneous exposure to hog barn dust and alcohol decreases inflammatory mediators, TNF-α, IL-6, and IL-8, in mice. Previously, mice exposed to both alcohol and hog barn dust showed a depleted amount of lymphocytes compared to mice exposed only to hog barn dust. Weakening of the innate immune response could lead to enhanced susceptibility to disease. In addition, mice that were co-exposed to hog barn dust and alcohol also experienced increased mortality.
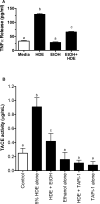
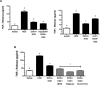
Methods: Because we recently demonstrated that PKA activation inhibits the TNF-α sheddase, TNF-α-converting enzyme (TACE), we hypothesized that an alcohol-mediated PKA pathway blocks TACE activity and prevents the normative inflammatory response to hog barn dust exposure. To delineate these effects, we used PKA pathway inhibitors (adenylyl cyclase [AC], cAMP, and PKA) to modulate the effects of alcohol on dust-stimulated TNF-α release in the bronchial epithelial cell line, BEAS-2B. Alcohol pretreatment blocked TACE activity and TNF-α release in hog barn dust-treated cells.
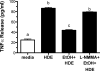
Results: Alcohol continued to block hog barn dust-mediated TNF-α release in the presence of the particulate AC inhibitor, SQ22,536. The soluble adenylyl cyclase inhibitor, KH7, however, significantly increased the inflammatory response to hog barn dust. phosphodiesterase 4 inhibitors significantly elevated cAMP and enhanced alcohol-mediated inhibition of dust-stimulated TNF-α release. In addition, the NO synthase inhibitor, l-NMMA, also reversed the alcohol-blocking effect on dust-stimulated TNF-α.
Conclusions: These data suggest that alcohol requires a soluble cyclase-generated cAMP-PKA pathway that is dependent upon the action of NO to inhibit TACE and TNF-α release. These findings support our observations that alcohol functions through a dual NO and PKA pathway in bronchial epithelial cells.
Keywords: Airways; Alcohol; Inflammation; Nitric Oxide; TNF-α-Converting Enzyme.
Copyright © 2016 by the Research Society on Alcoholism.
Figures
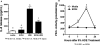
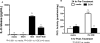



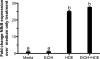

Similar articles
-
cAMP-dependent protein kinase activation decreases cytokine release in bronchial epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2014 Oct 15;307(8):L643-51. doi: 10.1152/ajplung.00373.2013. Epub 2014 Aug 22. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25150062 Free PMC article.
-
Expression of TNFalpha and its receptors R1 and R2 in human alveolar epithelial cells exposed to organic dust and the effects of 8-bromo-cAMP and protein kinase A modulation.Inflamm Res. 2005 Jul;54(7):281-8. doi: 10.1007/s00011-005-1356-7. Inflamm Res. 2005. PMID: 16134057
-
Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium.Alcohol Clin Exp Res. 1999 Sep;23(9):1528-33. Alcohol Clin Exp Res. 1999. PMID: 10512320
-
Bacterial-induced release of inflammatory mediators by bronchial epithelial cells.Eur Respir J. 1996 Sep;9(9):1913-22. doi: 10.1183/09031936.96.09091913. Eur Respir J. 1996. PMID: 8880112 Review.
-
Tumor necrosis factor alpha stimulation of human Clara cell secretory protein production by human airway epithelial cells.Ann N Y Acad Sci. 2000;923:193-201. doi: 10.1111/j.1749-6632.2000.tb05530.x. Ann N Y Acad Sci. 2000. PMID: 11193757 Review.
Cited by
-
Alcohol Inhibits Organic Dust-induced ICAM-1 Expression on Bronchial Epithelial Cells.Safety (Basel). 2017 Mar;3(1):5. doi: 10.3390/safety3010005. Epub 2017 Jan 7. Safety (Basel). 2017. PMID: 29082234 Free PMC article.
-
Sorrel Extract Reduces Oxidant Production in Airway Epithelial Cells Exposed to Swine Barn Dust Extract In Vitro.Mediators Inflamm. 2019 Aug 1;2019:7420468. doi: 10.1155/2019/7420468. eCollection 2019. Mediators Inflamm. 2019. PMID: 31481850 Free PMC article.
-
Recombinant CC16 protein inhibits the production of pro-inflammatory cytokines via NF-κB and p38 MAPK pathways in LPS-activated RAW264.7 macrophages.Acta Biochim Biophys Sin (Shanghai). 2017 May 1;49(5):435-443. doi: 10.1093/abbs/gmx020. Acta Biochim Biophys Sin (Shanghai). 2017. PMID: 28338974 Free PMC article.
-
The alcohol exposome.Alcohol. 2025 Feb;122:81-89. doi: 10.1016/j.alcohol.2024.12.003. Epub 2024 Dec 23. Alcohol. 2025. PMID: 39722409 Free PMC article. Review.
-
Dimethylarginine dimethylaminohydrolase (DDAH) overexpression enhances wound repair in airway epithelial cells exposed to agricultural organic dust.Inhal Toxicol. 2018 Feb;30(3):133-139. doi: 10.1080/08958378.2018.1474976. Epub 2018 May 25. Inhal Toxicol. 2018. PMID: 29793367 Free PMC article.
References
-
- Alterman T, Steege AL, Li J, Petersen MR, Muntaner C. Ethnic, racial, and gender variations in health among farm operators in the United States. Ann Epidemiol. 2008;18:179–186. - PubMed
-
- Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin Exp Res. 2003;27:1838–1845. - PubMed
-
- Brown LAS, Harris FL, Ping X, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. - PubMed
-
- Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:L824–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

